0574
Evolutionarily conserved fMRI network dynamics in the human, macaque and mouse brain1Functional Neuroimaging Laboratory,, Istituto Italiano di Tecnologia, CNCS, Rovereto, Italy, 2Neural Computation Laboratory, Istituto Italiano di Tecnologia, CNCS, Rovereto, Italy, 3Center for the Developing Brain, Child Mind Institute, New York, NY, United States
Synopsis
Recent work revealed that resting-state fMRI (rsfMRI) network dynamics in the mouse brain governed by infraslow transitions between a limited set of recurring BOLD co-activation patterns. Here we extend these findings to the macaque and awake human brain, showing that in both these higher mammalian species rsfMRI timeseries can be similarly deconstructed into a set of oscillatory coactivation patterns, whose occurrence is phase-locked to intrinsic global fMRI Signal (GS) fluctuations. Our results reveal a fundamental, evolutionarily conserved spatiotemporal structure of resting-state fMRI activity.
Introduction
Robust and reproducible patterns of spontaneous brain activity have been consistently mapped in humans using resting-state fMRI (rsfMRI)1,2,8. Back-translation of this method in primates and rodents has revealed evolutionarily conserved rsfMRI network topographies, including evolutionarily conserved precursors of distributed human networks such as the Default-Mode and salience networks3-5. Using frame-wise clustering, we demonstrated that spontaneous rsfMRI activity in the mouse brain is governed by recurrent transitions between a set of fluctuating BOLD co-activation patterns (CAPs). We also showed that CAPs are phase-coupled to intrinsic fluctuations in global fMRI signal (GS)6. It is however unclear whether such dynamic rules are species-specific, or reflect species-invariant dynamic generalizable principles across evolution in the mammalian brain. Here we expand our previous findings macaque and human brains showing that, as in rodents, a limited set of oscillating (CAPs) govern network dynamic also in higher mammalian species. Specifically, we report k=6-mice, 8-macaque, and 8-human reproducible CAPs, and show that these CAPs’ topographies encompass well-defined interactions between evolutionarily-relevant distributed rsfMRI network systems (e.g. DMN and task-positive networks). We also show that oscillatory CAP- anti-CAP transitions exhibit phase-locking to GS cycles in anesthetized conditions (mouse, monkey as well as awake human subjects. These results reveal a set of fundamental, evolutionarily-conserved principles that govern rsfMRI network dynamics in the mammalian brain.Methods
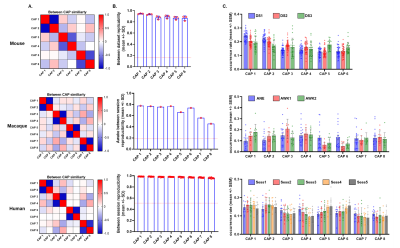
rsfMRI: Openly available data for mice6, macaques4, and humans2 were used. Briefly, we leveraged the availability of mouse-datasets (male C57BI6/J mice, n1=40–500 volumes, n2=41–300 volumes, and n3=21-45 volumes, all with TR=1.2s) acquired under halothane anesthesia (0.75%)6; Macaque experiments with one anesthetized dataset (Oxford, n=19-1600 volumes, TR=2s), and 2 test-retest datasets (New-Castle, n=10-250 volumes, TR=2s)4;and Five sessions from the Human Midnight-Scan-Club(n=10-813 volumes)2. Preprocessing: Data were pre-processed as previously described6:despiking; motion-correction; registration of anatomical and functional images to templates. Data was denoised by regressing 6 motion parameters and CSF and white matter signals, then band-pass filtered (0.01-0.1Hz), and smoothed. Data-analysis: Whole-brain BOLD transients were mapped by clustering fMRI frames into co-activation patterns(CAPs) exhibiting spatially congruent spontaneous BOLD-activity6. For the n=3 and n=5 datasets of macaque and humans, concatenated frames from all datasets were clustered according to their spatial-similarity using k-means++8, 1000-iterations and k=2:20. We incrementally assessed each solution based on its variance explained, and reproducibility of the identified CAPs in awake sessions for macaques, and humans6. For each species, clustered frames were voxel-wise averaged and normalized to T-scores. CAP map reproducibility was assessed by comparing the spatial similarity of group-averaged maps between independent datasets (mice) or sessions (awake macaques, and humans). CAP-dynamics: CAP-maps were projected to original rsfMRI-frames to obtain a “CAP time-series” for each subject, and their respective power-spectra were computed. CAP-occurrence within GS cycles was assessed by computing the GS’s instantaneous phase with the Hilbert-Transform. Circular-statistics was used to measure the probability distribution of GS-phases at each CAP’s occurrence6.Results
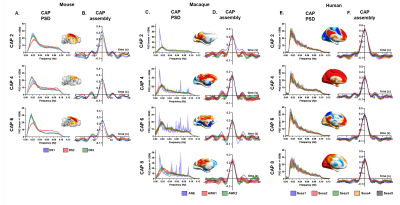
K-means clustering revealed that k=6 (mouse), and k=8 (monkey and human) provided a stable and reproducible partitions across datasets or repeated imaging sessions (Fig.1), explaining a large fraction of rsfMRI variance in all species (mouse:0.61; macaques:0.61, human:0.73). In keeping with prior mouse results, monkey and human CAPs could be clearly paired into spatially opposing CAP anti-CAP pairs. CAP topography mapping revealed evolutionarily conserved features across species (Fig. 2) including a competing engagement of default-mode (DMN) and somatosensory regions in CAPs 1-2 as well as wide pan-cortical patterns of coactivation in CAPs 3-4. Anesthetized mice and macaques shared concomitant engagement DMN and thalamic regions with converse co-activations of the hippocampus (CAPs 5-6), while in awake humans these three structures co-fluctuated. Finally, CAPs 7-8 in macaques and humans showed higher order networks (e.g. dorsal-attention) acting in unison with the DMN, TH, and primary visual cortices, while having converse co-activations in executive-control and salience Networks. Spectral analyses revealed that CAPs fluctuate with centered power in the infraslow range (0.01-0.04 Hz), with conserved assembly and disassembly dynamics (Fig.3). Finally, as previously shown in mice, CAP pairs in macaques and humans showed clear preferential occurrence within GS cycles across species also in awake conditions, as depicted by the distribution of infraslow GS phases at the occurrence of a CAP (Fig.4).Discussion and Conclusions
The observation of robust CAP anti-CAP configurations, and the fact that these recurring BOLD patterns explain a large portion of rsfMRI variance across species and states (anesthetized and awake) corroborate and expand previous investigation revealing a dominant effect of single peaks of BOLD fMRI activity to the establishment of network-wide fMRI connectivity9-12. The phase-coupling of these fluctuations to GS dynamics, and its extension to conscious conditions strongly supports emerging evidence suggesting a neural origin for this signal13, and putatively links these fluctuation to ascending modulatory activity and arousal14,15. In conclusion, our work describes a set dynamic features governing spontaneous fMRI activity in the mammalian brain, and substantiates a link between resting-state network activity and global fMRI fluctuations in the mammalian brain.Acknowledgements
This work has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (#DISCONN; no. 802371 to A.Gozzi.)References
1. Power, J.D., et al. (2014). Studying brain organization via spontaneous fMRI signal. Neuron 84, 681–696.
2. Gordon, E.M., et al. (2017). Precision Functional Mapping of Individual Human Brains. Neuron 95, 791-807.
3. Vincent, J.L., et al. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86.
4. Xu, T., et al. (2019). Interindividual Variability of Functional Connectivity in Awake and Anesthetized Rhesus Macaque Monkeys. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4:543–553.
5. Gozzi, A., and Schwarz, A.J. (2016). Large-scale functional connectivity networks in the rodent brain. Neuroimage 127, 496–509.
6. Gutierrez-Barragan, D., et al. (2019). Infraslow State Fluctuations Govern Spontaneous fMRI Network Dynamics. Current Biology, 29, 2295–2306.
7. Donahue, CJ, , et al. (2016). Using diffusion tractography to predict cortical connection strength and distance: A quantitative comparison with tracers in the monkey. J Neurosci 36:6758–6770.
8. Craddock, R. C., et al. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33(8), 1914–1928.
9. Abbas, A., et al. (2019). Quasi-periodic patterns contribute to functional connectivity in the brain. Neuroimage.191:193-204.
10. Liu, X., Duyn, J.H. (2013). Time-Varying Functional Network Information Extracted from Brief Instances of Spontaneous Brain Activity. Proc Natl Acad Sci U S A;110:4392–4397
11. Huang, Z., et al. (2020). Temporal circuit of macroscale dynamic brain activity supports human consciousness. Science Advances;6(11): eaaz0087.
12. Farnaz, Z.E., et al. (2020). High-amplitude cofluctuations in cortical activity drive functional connectivity. Proc Natl Acad Sci U S A; 117 (45) 28393-28401
13. Schölvinck, M.L., et al. (2010). Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. USA; 107: 10238-10243
14. Liu, X., et. al (2018). Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. 9, 395.
15. Chang, C., et al. (2016). Tracking brain arousal fluctuations with fMRI. Proc. Natl. Acad. Sci. USA; 113: 4518-4523
Figures