0573
Evolutionary gap of the default mode network organization between non-hominid primates and humans1Neurobiology and Anatomy, Wake Forest University, Winston Salem, NC, United States, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, University of Western Ontario, London, ON, Canada, 3Department of Physiology and Pharmacology, The University of Western Ontario, London, ON, Canada, 4Department of Radiology, Section of Neuroradiology, Wake Forest University, Winston Salem, NC, United States, 5Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, United States, 6Neurodegenerative Diseases Laboratory, Centre National de la Recherche Scientifique (CNRS), Université Paris-Sud, Université Paris-Saclay UMR 9199, Fontenay-aux-Roses, France, 7Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Direction de la Recherche Fondamentale (DRF), Institut François Jacob, MIRCen, Fontenay-aux-Roses, France, 8Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
We performed cross-species comparison to determine how the human default mode network (DMN) connectivity pattern compares to non-hominid primates. We characterized and compared the resting-state network functional organisation in humans, macaques, marmosets, and mouse lemurs using functional and anatomical atlases. We found decreased engagement of mPFC (medial prefrontal cortex) in all non-hominid primates “DMN-like” compared to humans. Another network involving mPFC was identified in all non-hominid primates but not in humans. Altogether, our results show that high order networks often assumed to be shared across primates diverge considerably between non-hominid species and humans.
Introduction
The default mode network (DMN) is unique in supporting internal mental processes in humans. Its deactivation is critical for engaging in cognitive processes (Raichle et al., 2001). An homologous network to the DMN was proposed in multiple non-human primate species [1-4]. Despite the similarities of the human and non-human primate DMN, qualitative differences are also consistently present in non-human primate studies [5]. The mPFC is deemed to support a gateway function in self representation in humans [6]. However, in most non-human primates, the mPFC has not been found to play a role in the DMN undermining the proposed role of the primate DMN in self-directed thought or introspection. Only in chimpanzees, has a DMN been defined where fMRI found that medial prefrontal regions were strongly connected to the PCC as in humans [7]. Together, these studies suggest a possible evolutionary gap between hominid and non-hominid primates (NHoPs) DMN connectivity, especially in the medial prefrontal area.Methods
Fourteen anaesthetized mouse lemurs were scanned using an EPI sequence on an 11.7T [4]. Four common marmosets were scanned awake and anesthetized on a 9.4T [8] and thirteen anaesthetized macaques were scanned on 3T Siemens MAGNETOM Skyra scanner. Forty-eight healthy humans resting-state fMRI images were generated by Castellanos et al. [9]. Images from each species were co-registered to a standardize space (AFNI) for statistical analyses [10].Results
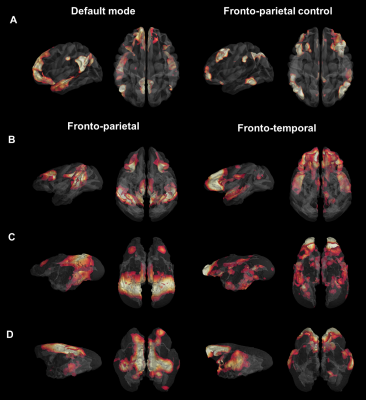
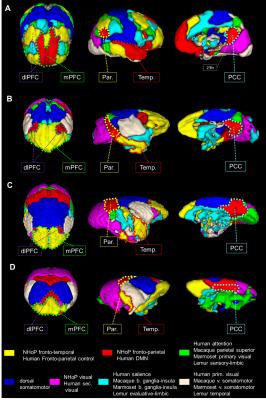
We first used identical group dictionary analysis on fMRI data and seven components were set for all analyses to extract similar networks as in the functional atlas of Yeo et al. [11]. For each species, the seven large-scale networks were labelled according to the literature and to their anatomical features (Fig.1). In humans, we extracted networks identified in the literature as the DMN and the fronto-parietal network (FPN) (Fig. 1A). In macaques, the same analysis revealed a cortical network encompassing mainly PCC (posterior cingulate cortex), parietal, and dlPFC (dorso-lateral prefrontal cortex) areas identified as the FPN (Fig. 1B) as well as a network involving mPFC and areas surrounding superior temporal sulcus identified as the fronto-temporal network (FTN). In marmosets, we extracted a component encompassing PCC, parietal, and dlPFC areas identified as the fronto-parietal network (FPN; Fig. 1C). We also found evidence for the FTN encompassing the mPFC and temporal areas (Fig. 1C). In mouse lemurs, we determined FPN from a cortical network encompassing the PCC, the parietal posterior cortex, and anterior lateral frontal regions (Fig. 1D) and FTN encompassing mPFC, dlPFC, and the middle temporal cortex (Fig. 1D). Each large-scale network was transformed into a mask and concatenated together, producing a 3D atlas for each species (Fig. 2, each atlas can freely be downloaded at http://www.nitrc.org/projects/prim_func_2020/). Cerebral clusters were spatially separated and attributed to a unique label.Fingerprint comparison
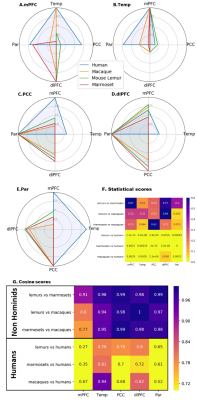
Fingerprint analysis was performed on key clusters of the fronto-parietal network/DMN and functional connectivity between these clusters was calculated using correlation coefficients. The mean connectivity matrix between the five clusters identified as Temporal (Temp, Fig. 3B), PCC (Fig. 3C), dlPFC (Fig. 3D), and Parietal (Par, Fig. 3E) was performed. Cosine similarity can be used as an index indicating the degree of similarity between two fingerprints. Permutation testing was performed on the cosine similarity indexes. A low cosine similarity associated to low p-value (p<0.05) suggests differences in connectivity profiles. We compared the fingerprints using permutation cosine similarity on similar clusters and between every pairs of species. NHoPs showed greater cosine similarity than humans (Fig 3. F-G).
Comparison of pairwise correlations
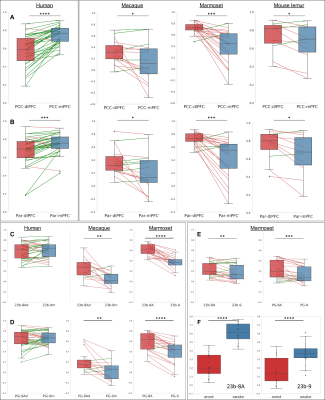
We performed a pairwise comparison of the relative differences between functional clusters of interest (Fig 2). We established that in macaques, marmosets, and mouse lemurs, the functional connection between PCC-mPFC was lower than between PCC-dlPFC (Fig. 4A) and the opposite relationship was observed in humans (Fig. 4A). This opposite relationship was also confirmed by comparing the correlation coefficients between Par-mPFC and Par-dlPFC (Fig. 4 B). To increase the spatial accuracy of the pairwise comparison, we replicated the analysis using three anatomical atlases from the literature [12-14]. Similar results were obtained, except that in humans, connectivity between mPFC (area 9) and dlPFC (8Ad) to PCC (23b) were not statistically different (Fig. 4C). To control for possible effects of anaesthesia, which some animals received, we compared their results with animals who did not receive anaesthesia. We found that relative differences of connectivity measured between mPFC, dlPFC to PCC (23b-9; 23b-8A) under anaesthesia, while still present were somewhat underestimated.
Conclusion
Identification of a common network anatomical architecture as well as functional connections supports the proposal that evolutionary rules apply distinctly to humans and NHoPs. First, the robust connection between mPFC-PCC was found to be unique to human (or hominid) specialization. Second, area 8Ad was found to be a common/ancestral frontal area of all primates’ DMN(-like). Globally, characteristics of high order networks in all non-hominid primate species examined are closer to each other than to humans. These new evolutionary rules of the DMN connectivity could open new research areas to explain the cognitive gap between hominid and non-hominid primates as well as improve the use of meaningful translational models of neuropathology.Acknowledgements
No acknowledgement found.References
1. Barks, S.K., L.A. Parr, and J.K. Rilling, The default mode network in chimpanzees (Pan troglodytes) is similar to that of humans. Cerebral cortex (New York, N.Y. : 1991), 2015. 25(2): p. 538-544.
2. Vincent, J.L., et al., Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 2007. 447(7140): p. 83-6.
3. Belcher, A., et al., Large-Scale Brain Networks in the Awake, Truly Resting Marmoset Monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2013. 33: p. 16796-804.
4. Garin, C.M., et al., Resting state functional atlas and cerebral networks in mouse lemur primates at 11.7 Tesla. NeuroImage, 2020: p. 117589.
5. Liu, C., et al., Anatomical and functional investigation of the marmoset default mode network. Nature Communications, 2019. 10(1): p. 1975.
6. Davey, C.G., J. Pujol, and B.J. Harrison, Mapping the self in the brain's default mode network. NeuroImage, 2016. 132: p. 390-397.
7. Barks, S.K., L.A. Parr, and J.K. Rilling, The default mode network in chimpanzees (Pan troglodytes) is similar to that of humans. Cereb Cortex, 2015. 25(2): p. 538-44.
8. Hori, Y., et al., Altered Resting-State Functional Connectivity Between Awake and Isoflurane Anesthetized Marmosets. Cerebral Cortex, 2020. 30(11): p. 5943-5959.
9. Castellanos, F.X., C. Kelly, and M.P. Milham, The restless brain: attention-deficit hyperactivity disorder, resting-state functional connectivity, and intrasubject variability. Can J Psychiatry, 2009. 54(10): p. 665-72.
10. Cox, R.W., AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 1996. 29(3): p. 162-73.
11. Yeo, B.T.T., et al., The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology, 2011. 106(3): p. 1125-1165.
12. Glasser, M.F., et al., A multi-modal parcellation of human cerebral cortex. Nature, 2016. 536(7615): p. 171-178.
13. Reveley, C., et al., Three-Dimensional Digital Template Atlas of the Macaque Brain. Cerebral cortex (New York, N.Y. : 1991), 2017. 27(9): p. 4463-4477.
14. Liu, C., et al., A digital 3D atlas of the marmoset brain based on multi-modal MRI. Neuroimage, 2018. 169: p. 106-116.
Figures