0566
Wireless Body Sensor Data Acquisition Platform for Motion Tracking1Center for Biomedical Imaging, NYU Grossman School of Medicine, New York City, NY, United States, 2Center for Advanced Imaging Innovation and Research, NYU Grossman School of Medicine, New York City, NY, United States
Synopsis
The temporal resolution in MRI can be too slow to track respiratory or bulk subject motion. Auxiliary sensors have been developed to track motion with high temporal resolution, but can require cumbersome cabling. In this work, we describe an MRI-compatible wireless accelerometer data acquisition platform and demonstrate proof-of-concept by correlating respiratory motion data with independent, ground-truth optical measurements.
Introduction
Clinical MRI of the abdomen provides a range of diagnostic information but its temporal resolution can be too slow to track respiratory or bulk subject motion. Tracking methods that rely solely on k-space data can fail in the event of irregular, unexpected motion, while MR navigators require customized pulse sequences that typically prolong scan time. Auxiliary sensors (1-4), such as a pneumatic belt (5), can track motion with high temporal resolution but can lack robustness because of position dependency. Recent work demonstrated that a three-axis accelerometer provides signals representing respiratory motion (6). Such sensors, while also subject to positional variability, are prime candidates to form a multi-sensor array, where in principle ambiguities from spurious data from one sensor can be resolved with credible data from neighboring sensors. However, cabling is cumbersome and has the potential to interfere with native electromagnetic fields. In this work, we describe a wireless sensor acquisition platform (7) and demonstrate proof-of-concept with a single sensor. We report on its compatibility with a 0.55T MRI system, describe a pneumatic motion phantom from which “ground-truth” optical tracking measurements are derived, show correlation between ground-truth and wirelessly acquired accelerometer data, and discuss steps needed to scale-up the platform.Methods
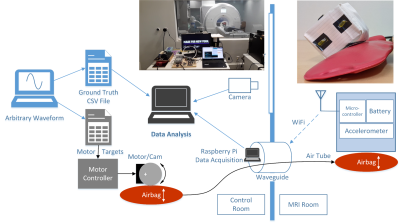
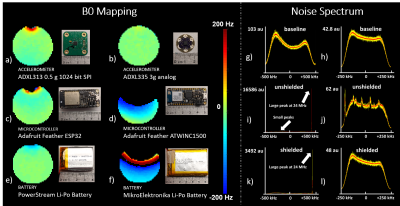
We tested accelerometers, microcontrollers, and batteries for compatibility with a prototype Aera MAGNETOM MRI scanner that was ramped down to 0.55T (Siemens Healthcare, Erlangen, Germany). Selected components were used to build a wireless motion-tracking module that was enclosed in a copper box to reduce RF interference and wrapped in a flame resistant fabric for safety. The module utilized 2.4GHz WiFi to transfer data to a remote Raspberry Pi (Figure 1). Initial tests confirmed the absence of significant ferromagnetic or Lorentz forces on the module and that its temperature remained within 1°C of its baseline value during 10 minute RF- and gradient-intensive scans. To determine whether accelerometer signals were representative of motion and if they could be reliably sent wirelessly from the MRI environment, we built an MRI-compatible phantom (8-12) to mimic respiratory motion. We drove the phantom with a stepper motor (13) that mechanically compressed an air bladder within the control room that was connected to an identical counterpart in the scan room. Predefined waveforms were fed to the motor via an Arduino based controller. To account for discrepancies due to non-linearities inherent in pneumatic systems, we implemented an independent optical-based tracking system that utilized fiducial markers attached to the sensor module as a secondary “ground-truth” (14). This optical tracking system also provided ground-truth data for in-vivo experiments, where perfect waveforms cannot be prescribed. One subject was scanned after supplying informed written consent per our local internal review board. The wireless sensor module was placed on the chest, distal to the sternum. Data were acquired while the subject performed normal and fast breathing with the scanner idle and during a gradient echo sequence for abdominal MRI.Results
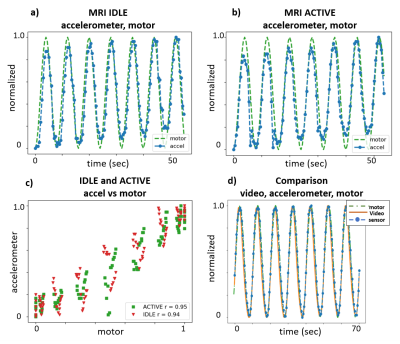
Frequency maps show susceptibility distortion from various components, indicating that component choice plays a role in module layout and placement in the MRI FOV (Figure 2). While RF noise spectra show the wireless device did not interfere with the MRI at 23.5±0.25MHz, we observed a spike at 24MHz that was likely a low order harmonic of the 2.4GHz WiFi. In phantom experiments, the idealized motor waveform, ground truth video, and accelerometer measurements were well-correlated, indicating that the accelerometer provides relevant motion information and that RF and gradient pulses do not interfere with the wireless device (Figure 3). In vivo data show excellent correlation between video and accelerometer measurements during normal (~0.25Hz) and fast (~0.8Hz) respiration (Figure 4).Discussion and Conclusion
We presented a wireless acquisition system for motion tracking and demonstrated its MRI compatibility through RF noise, temperature, and magnetic field measurements, and its relevance to motion prediction by correlating optical “ground truth” motion data. The system was built primarily using low cost, off-the-shelf components and controlled with open-source hardware and software to facilitate future dissemination. While off-the-shelf devices provided a convenient starting point, a large-scale array may require customized PCBs to miniaturize the system and minimize susceptibility artifacts. This work shows proof-of-concept for wirelessly transferring accelerometer data that is well correlated with normal and fast breathing motion. More work is needed to optimally utilize multi-axis information from the accelerometer, to determine a proper sampling rate sufficient to capture irregular motion, and finally to develop a pixel-wise motion model, as is needed for MRI motion correction. To help address these issues, we plan to expand the individual sensor system into a multi-channel array that could potentially overcome sampling limitations and whose localized measurements could be used to establish fingerprints corresponding to respiratory, cardiac, and bulk motion, for example. Such an array could be cumbersome to implement in a wired fashion and would benefit from the proposed wireless system. In conclusion, we demonstrate a system for wireless transfer of accelerometer-derived motion information and show that it correlates with ground truth measurements. We point out that the wireless system can serve as a foundation for sensors other than accelerometers and anticipate that it will prove useful in the quest for free-breathing, motion-robust MRI.Acknowledgements
The authors thank Jan Paska, Christopher Collins, Inge Brinkmann and Mahesh Bharath (Siemens Medical Solutions), and Lukas Winter (Physikalisch-Technische Bundesanstalt) for discussions on sensors and MRI compatible devices and Justin Ho for mechanical assistance. This work was partially supported by National Institutes of Health grants R21CA213169, R01DK106292, R21AG061579, R01DK114428, and R21EB027263 and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at the New York University School of Medicine, which is an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
1. Preiswerk F, Toews M, Cheng CC, Chiou JG, Mei CS, Schaefer LF, Hoge WS, Schwartz BM, Panych LP, Madore B. Hybrid MRI-Ultrasound acquisitions, and scannerless real-time imaging. Magn Reson Med 2017;78(3):897-908.
2. Speck O, Hennig J, Zaitsev M. Prospective real-time slice-by-slice motion correction for fMRI in freely moving subjects. Magma 2006;19(2):55-61.
3. Forman C, Aksoy M, Hornegger J, Bammer R. Self-encoded marker for optical prospective head motion correction in MRI. Med Image Anal 2011;15(5):708-719.
4. Auboiroux V, Petrusca L, Viallon M, Muller A, Terraz S, Breguet R, Montet X, Becker CD, Salomir R. Respiratory-gated MRgHIFU in upper abdomen using an MR-compatible in-bore digital camera. Biomed Res Int 2014;2014:421726.
5. Henningsson M, Botnar RM. Advanced respiratory motion compensation for coronary MR angiography. Sensors 2013;13(6):6882-6899.
6. Chen B, Weber N, Odille F, Large-Dessale C, Delmas A, Bonnemains L, Felblinger J. Design and Validation of a Novel MR-Compatible Sensor for Respiratory Motion Modeling and Correction. IEEE Trans Biomed Eng 2017;64(1):123-133.
7. Sengupta S, Tadanki S, Gore JC, Welch EB. Prospective real-time head motion correction using inductively coupled wireless NMR probes. Magn Reson Med 2014;72(4):971-985.
8. Bolwin K, Czekalla B, Frohwein LJ, Buther F, Schafers KP. Anthropomorphic thorax phantom for cardio-respiratory motion simulation in tomographic imaging. Phys Med Biol 2018;63(3):035009.
9. De Tillieux P, Topfer R, Foias A, Leroux I, El Maachi I, Leblond H, Stikov N, Cohen-Adad J. A pneumatic phantom for mimicking respiration-induced artifacts in spinal MRI. Magn Reson Med 2018;79(1):600-605.
10. Drangova M, Bowman B, Pelc N. Physiologic motion phantom for MRI applications. J Magn Reson Imaging 1996;6(3):513-518.
11. Tavallaei MA, Johnson PM, Liu J, Drangova M. Design and evaluation of an MRI-compatible linear motion stage. Med Phys 2016;43(1):62.
12. Nofiele J, Yuan Q, Kazem M, Tatebe K, Torres Q, Sawant A, Pedrosa I, Chopra R. An MRI-compatible platform for one-dimensional motion management studies in MRI. Magn Reson Med 2016;76(2):702-712.
13. Han H, Moritz R, Oberacker E, Waiczies H, Niendorf T, Winter L. Open Source 3D Multipurpose Measurement System with Submillimetre Fidelity and First Application in Magnetic Resonance. Sci Rep 2017;7(1):13452.
14. Brown D. Tracker video analysis. 2004. https://physlets.org/tracker/
Figures