0555
Combined multiparametric high resolution diffusion-relaxometry on 7T (OKAPI)1Centre for Medical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
A multi-parametric quantitative MRI sequence called ZEBRA, which varies diffusion weighting, inversion time (TI) and gradient echo time (TE) by shuffling flexibly diffusion preparation, slice acquisition order, slice spacing and diffusion properties and by adding multiple gradient echo read-outs sharing one diffusion preparation is explored on a 7T scanner in high resolution.
Introduction
Diffusion MRI (dMRI) and relaxometry are both widely used to allow quantitative insights into tissue structure and function. Changing different properties of the diffusion preparation (b-value and b-vector) makes the dMRI acquisition sensitive to different scales and directions of microstructure and supports calculation of absolute quantities. Similarly, varying the echo time (TE) and inversion time (TI) among others allows the decay and recovery of transverse and longitudinal magnetization to be sampled, and thus extract quantitative T2/T2* and T1 maps. Recent advances focusing on combining both diffusion and relaxometry data in joint analysis [Veraart2017,Slator2018] have shown the potential for novel insights [DeSantis2016]. However, due to the enlarged parameter space required, efficient acquisition techniques are required and have been proposed for example with the ZEBRA technique [Hutter2018].In parallel, the promise of increased signal has been driving novel developments at higher field strength [Gallaghan2018]. However, high field imaging poses specific challenges particularly for dMRI: these include the need to be overcome shorter T2 times which could potentially cancel some of the benefits of increased signal; increased main magnetic field (B0-field)-inhomogeneity particularly relevant due to the often used EPI imaging for dMRI and increased B1-inhomogeneity, particularly relevant due to the employed spin echo sequences for dMRI. Finally, the longer T1 and SAR limits render scanning at high field inefficient, particularly increasing the need for more efficient techniques.This study therefore explores the efficient multi-dimensional sequence ZEBRA on high field in high resolution as a first step towards multi-dimensional quantitative experiments in the described larger parameter space exploiting the benefits of high field.Methods
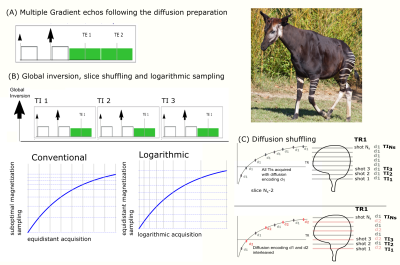
A single-shot Echo-Planar Imaging sequence with pulsed gradient diffusion weighting was modified to enable efficient acquisition of the multi-dimensional parameter space, previously presented as the ZEBRA method [Hutter2018]. The echo train was extended by adding up to two echos sampling the decay of transverse relaxation (See Figure 1A). A global adiabatic pulse was added before the first excitation during each TR to allow sampling of the magnetization recovery curve. To ensure that all slices are acquired with the required TI combinations, the order of the geometric locations being excited is shuffled freely as initially proposed by Ordidge et al [Ordidge1990] (See Figure 1B). Finally, the temporal spacing of the excitations within each TR - traditionally chosen as equidistantly spaced - was modified to logarithmic spacing enabling uniform sampling of the exponential magnetization recovery curve. To increase the efficiency of the aforementioned slice shuffling, the chosen diffusion preparation was modified for every slice instead of every volume allowing subsampling of the recovery curve (See Figure 1C).Data with the ZEBRA sequence was acquired on three healthy volunteers on a Siemens 7T Terra pTx system. The final protocol as illustrated below had the following parameters: FOV=220x220mm, slice thickness 2mm, resolution 1.25x1.25x2mm, TR=5s, TE= [72ms,100ms,140ms], TA=4:05min. Partial Fourier 5 / 8, GRAPPA parallel imaging acceleration factor 3, number of slices 12, phase encoding direction AP and PA. Twelve diffusion directions were acquired at b=1000 (8) and b=0 (4) and twelve different TIs - given by the number of excitations and thus slices per TR were obtained. The sequence gives flexibility regarding the exact shuffling pattern in both slice and diffusion preparation as well as in the parametrization of the logarithmic sampling. For the present experiment, a target T1 - corresponding to the recovery curve sampled most efficiently - of 1000ms was chosen.Results
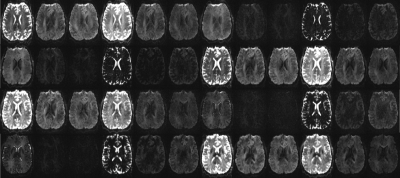
144 unique contrasts were acquired for all slices in 4min, modifying TI, TE and diffusion properties as described above. Figure 2 shows data acquired with the sequence for all considered sequence parameters for one slice, located mid-brain, illustrating the variety of contrasts achieved. The high resolution allows details such as the periventricular white matter to be well resolved.Discussion and Conclusion
Exploration of a combined diffusion-relaxometry acquisition was successfully demonstrated on 7T with initial results from healthy volunteers. Next steps include the optimization of the acquisition protocol and choice of samples, for example by including different tensor shapes. Furthermore, in line with the above mentioned specific high field challenges, sampling spin and gradient echos within <100ms allows, as shown before [Cordero-Grande2018], dynamic and high quality resolution of the distortion artifacts. This will be explored on the high field data. Dedicated analysis techniques will be explored.Acknowledgements
This work was supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London [WT 203148/Z/16/Z], Wellcome Trust Sir Henry Wellcome Fellowship [201374/Z/16/Z], UKRI FLF [MR/T018119/1] and Open Science Enrichment award. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The authors thank all involved radiographers and volunteers for their invaluable help.References
[Gallaghan2018] Neuroimage, 2018 Mar;168:172-180. doi: 10.1016/j.neuroimage.2017.04.037, Diffusion MRI of the human brain at ultra-high field (UHF): A review
[Hutter2018] Integrated and efficient diffusion-relaxometry using ZEBRAJ Hutter, P J. Slator, D Christiaens, R PAG Teixeira, T Roberts, L Jackson, A N. Price, S Malik, J V. Hajnal, Scientific Reports 2018: 15138
[Cordero-Grande2018] SAFE, ISMRM 2018
[DeSantis2016] De Santis, S., Assaf, Y., Jeurissen, B., Jones, D. K. & Roebroeck, A. T 1 relaxometry of crossing fibres in the human brain. NeuroImage 141, 133–142, https://doi.org/10.1016/j.neuroimage.2016.07.037 (2016).
[Ordidge1990] Ordidge, R. J., Gibbs, P., Chapman, B., Stehling, M. K. & Mansfield, P. High-speed multislice T1 mapping using inversion-recovery echo-planar imaging. Magn. resonance medicine 16, 238–45 (1990).
Figures