0547
Dysfunction of Olfactory Resting-State Functional network in Early-onset Early-stage Parkinson’s Disease
Jianli Wang1, Rachel Stanford1, Lauren Spreen2, Jeffrey Vesek2, Christopher Sica1, Thyagarajan Subramanian3, and Qing X Yang4
1Radiology, Penn State College of Medicine, HERSHEY, PA, United States, 2Molecular Biology, Penn State College of Medicine, HERSHEY, PA, United States, 3Neurology, Penn State College of Medicine, HERSHEY, PA, United States, 4Neurosurgery, Penn State College of Medicine, HERSHEY, PA, United States
1Radiology, Penn State College of Medicine, HERSHEY, PA, United States, 2Molecular Biology, Penn State College of Medicine, HERSHEY, PA, United States, 3Neurology, Penn State College of Medicine, HERSHEY, PA, United States, 4Neurosurgery, Penn State College of Medicine, HERSHEY, PA, United States
Synopsis
Hyposmia is prevalent in Parkinson’s disease (PD) and the central olfactory system is highly affected by PD pathology. Despite the considerable progresses in understanding the pathophysiology of the disease, the mechanism causing hyposmia in PD is still unclear. Given that there is early PD-related neurodegeneration in anterior olfactory nucleus, which is a part of the primary olfactory cortex, we tested the hypothesis that there are PD-related dysfunctions in the central olfactory functional network at the early stage of disease.
Introduction
Hyposmia has been reported to occur in the majority of early-stage Parkinson’s disease (PD)1-3. The central olfactory system is highly affected by PD pathology 4-6. Postmortem studies have found significant neuronal loss in the central olfactory structures, and Lewy pathology, the marker for PD, initiates in the olfactory bulb (OB) and anterior olfactory nucleus (AON) 4, which occurs approximately 4 years earlier than in the SN 7, 8. Despite these considerable progresses in understanding the pathophysiology of the disease, the mechanism causing hyposmia in PD is still unclear. Given that there is early PD-related neurodegeneration in AON, which is a part of the primary olfactory cortex (POC)3, 5, we hypothesized that there are PD-related dysfunction in the central olfactory functional network at the early stage of disease. To test this hypothesis, we studied the changes of olfactory resting-state functional network in early-stage PD subjects. Furthermore, we sought to determine the relevance between such changes and smell deficits.Methods
Human Subjects Forty-three early-onset early-stage (H&Y stage 1-2) cognitively normal idiopathic PD subjects participate in the study (Table 1). Their motor function deficits were evaluated with the Part 3 of Unified Parkinson’s Disease Rating Scale (UPDRS-III). For comparison, 27 age/sex-matched healthy subjects participated as healthy controls (HC). There was no significant difference in the age, sex, educational level, and cognitive function (Montreal Cognitive Assessment, MoCA) between the two groups. Psychophysical testing was performed on all the subjects for their smell identification function and smell threshold using the OLFACT Test Battery (Osmic Enterprises, Inc).Resting-state fMRI (rsfMRI) rsfMRI was conducted with the subjects eyes open on a Siemens 3 T scanner with a 64-channel head-neck coil and a blood-oxygen-level-dependent (BOLD) signal sensitive T2*-weighted EPI sequence using optimized spectral-spatial radiofrequency pulses (SPSP) for through-plane compensations to effectively recover the diminished fMRI signal in some central olfactory regions, e.g., orbitofrontal cortex (OFC), which are in close proximity to air/tissue boundaries and suffer from susceptibility-related MR signal losses 9. The scan parameters were: repetition time = 2000 ms, echo time = 30 ms, field of view = 220 mm × 220 mm, acquisition matrix = 80 mm × 80 mm, image resolution = 2.8 mm × 2.8 mm, slice thickness = 4 mm, 34 oblique slices, accelerating factor (GRAPPA iPAT) = 2, acquisition time = 5 min.
Data Analysis The functional connectivity (FC) of the central olfactory network was estimated using Data Processing Assistant for Resting-State fMRI (http://rfmri.org/DPARSF) with bilateral POC (include anterior olfactory nucleus, piriform cortex, periamygdaloid cortex, anterior entorhinal cortex) as seeds. The comparisons between the FCs in the HC and PD subjects was conducted using 2-sample t-tests. The correlations between FC with the smell functions were evaluated using multiple regressions.
Results
Psychophysical tests showed significant olfactory functional deficits in the PD subjects with smell identification score of 13.2 ± 3.7 and smell threshold of 5.7 ± 2.9, both were significantly lower than the HCs (smell identification score 18.7 ± 2.4 and smell threshold 9.2 ± 3.2) (two sample t-tests, p = 2.2×10-9 and 0.00001, respectively). The major structures having strong FCs with bilateral POCs in both HC and PD groups were the OFC, hippocampus and parahippocampal gyrus, and anterior cingulate cortex (FWE, p < 0.05, extent threshold = 6 voxels). However, the FCs with bilateral POCs in the left substantia nigra (SN), temporal pole, interior temporal gyrus, insular cortex, and posterior hippocampus and parahippocampal gyrus of PDs were significantly weaker than the HCs (2-sample t-test, uncorrected, p < 0.005, extent threshold = 6 voxels) (Fig. 1). There were significant positive correlations between the smell threshold scores and FCs in those structures of all the subjects, however, within the PD group these correlations were not significant. There was no significant correlation between the UPDRS scores and the FCs in the central olfactory structures.Discussion
In this study, the olfactory resting-state functional network in both HC and PD was identified and evaluated. In early-stage PD, the resting-state FC between POC and some structures in the network was significantly weaker than that in the HCs, and the lowered FC correlated with the deficits in smell threshold. These results support our hypothesis of PD-related dysfunction in the central olfactory functional network at the early stage of disease. In addition, the finding of lowered FC between POC and SN in early-stage PD suggests the involvement of the dopaminergic system in the dysfunction of the central olfactory network. All these findings are important for understanding the mechanisms underlying olfactory deficits in PD.Acknowledgements
This study was supported by the DANA Foundation and NIH R01NS099630.References
- Haehner A, et al. Prevalence of smell loss in Parkinson's disease--a multicenter study, Parkinsonism Relat Disord 2009, 15:490-494.
- Muller A, et al. Olfactory function in Parkinsonian syndromes, J Clin Neurosci 2002, 9:521-524.
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration, Neurology 1988, 38:1237-1244.
- Braak
H, et al. Staging of
the intracerebral inclusion body pathology associated with idiopathic
Parkinson's disease (preclinical and clinical stages), J Neurol 2002, 249 Suppl
3:III/1-5.
- Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson's disease, Mov Disord 1995, 10:283-287.
- Harding AJ, et al. Clinical correlates of selective pathology in the amygdala of patients with Parkinson's disease, Brain 2002, 125:2431-2445.
- Berendse HW, Ponsen MM. Detection of preclinical Parkinson's disease along the olfactory trac(t), J Neural Transm Suppl 2006, 321-325.
- Haehner
A, et al. Olfactory loss may
be a first sign of idiopathic Parkinson's disease, Mov Disord 2007, 22:839-842.
- Yip CY, et al. Spectral-spatial pulse design for through-plane phase precompensatory slice selection in T2*-weighted functional MRI. Magn Reson Med, 2009. 61:1137-1147.
Figures
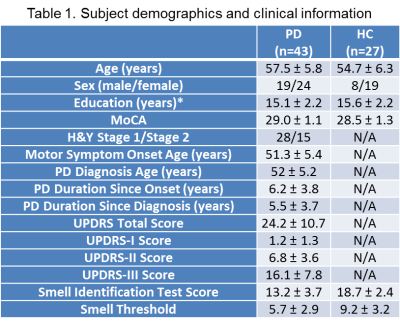
Values
are
presented in mean ± SD. *, p <
0.05, MoCA,
Montreal Cognitive Assessment; UPDRS, Unified Parkinson’s Disease Rating Scale;
N/A, not applicable.

Figure 1. Significantly
weaker FC with bilateral POCs in the left substantia nigra (SN), temporal pole,
interior temporal gyrus, insular cortex, and posterior hippocampus and parahippocampal
gyrus of PDs (2-sample t-test, uncorrected, p < 0.005, extent threshold = 6
voxels)