0543
Tract Density Imaging in Patients with Parkinson’s Disease Before and After Magnetic Resonance-guided Focused Ultrasound1Department of Medical Imaging, Henan Provincial People’s Hospital & Zhengzhou University, Zhengzhou, China, 2MR Collaboration, Siemens Healthcare Ltd. Beijing China, Beijing, China
Synopsis
Magnetic resonance-guided focused ultrasound (MRgFUS) is a non-invasive tremor therapy associated with Parkinson disease (PD). Conventional fractional anisotropy analysis might neglect pre- and postoperative microanatomic changes due to low spatial resolution. Finer brain structural depictions could be provided using track density imaging (TDI), a super-resolution reconstruction method. This study conducted TDI on seven patients with PD before and 1-month after MRgFUS and found that tract density values were significantly decreased postoperatively in the genu of the corpus callosum and left globus pallidus. TDI could be a valuable tool for detecting microstructural changes after MRgFUS therapy.
Introduction
Tremor is a common symptom in patients with Parkinson’s disease (PD). The dentato-rubro-thalamo-cortical (DRTC) pathway is a common component in tremor pathogenesis that comprises several important nuclei, such as the cerebellar dentate nucleus, red nucleus, thalamic ventral intermediate (VIM) nucleus, and connected white-matter fibers. VIM nucleus thalamotomy using magnetic resonance-guided focused ultrasound (MRgFUS) is a non-invasive procedure for treating medication-refractory tremor in PD. The mechanism by which MRgFUS thalamotomy mitigates tremors remains unclear; however, evidence has shown that MRgFUS could result in short- and long-term white-matter changes using diffusion tensor imaging [1]. Super-resolution track density imaging (TDI) is a reconstruction method that exploits the inherently high directional accuracy of diffusion fiber tractography to generate anatomic images able to depict overall changes in fiber tract numbers within each voxel in gray- and white-matter anatomy with sub-millimeter voxel resolution [2,3,4]. Thus, the aim of this study was to use TDI to evaluate microstructural changes in the brain of patients with tremor-dominant PD after MRgFUS therapy.Methods
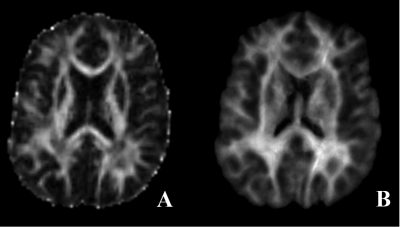
Seven patients with PD (5 men and 2 women; age range: 48–64 years) were enrolled. Five and two patients were ablated in the left and right sides of the thalamus, respectively. All patients underwent MRI preoperatively and 1 month after MRgFUS therapy. MR imaging (MRI) data were acquired on a 3T MAGNETOM Prisma scanner (Siemens Healthcare, Erlangen, Germany) equipped with a 64-channel head/neck coil. The protocol included MP2RAGE and diffusion MR sequence. The parameters for diffusion MRI were as follows: repetition time = 3300 ms, echo time = 64 ms, field of view = 207 × 207mm2, number of slices = 69, voxel size = 2.2 × 2.2 × 2.2 mm3, b value = 0 and 3000 sec/mm2, directions = 64. The diffusion images were processed to generate super-resolution TDI maps with 30 million fiber bundles using MRtrix (https://www.mrtrix.org). The processing steps included eddy current distortion corrections, fiber orientation distribution estimations, and probabilistic tractography. Finally, TDI maps were obtained at a high spatial resolution of 1.0 × 1.0 × 1.0mm3 (Figure 1). For the two patients whose ablated point was on the right side of the thalamus, the TDI data were left and right reversed so that the TDI data could be assessed in a single group to explore the effects of surgical treatment. Specifically, TDI was non-linearly registered to the FMRIB58_FA template in the Montreal Neurological Institute space using ANTS software (http://www.picsl.upenn.edu/ANTS/) [5] with the help of T1 MP2RAGE segmentation.Voxel-based analyses using SPM12 software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12) was used for whole-brain analyses of the TDI data to explore the pre- and postoperative differences. A paired-samples T-test was used. The value was reported in the form of mean±standard deviation. Additionally,the potential changes in fractional anisotropy (FA) were also analyzed using the tract-based spatial statistics method.Results
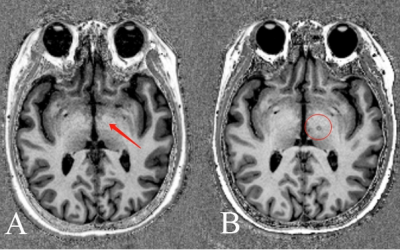
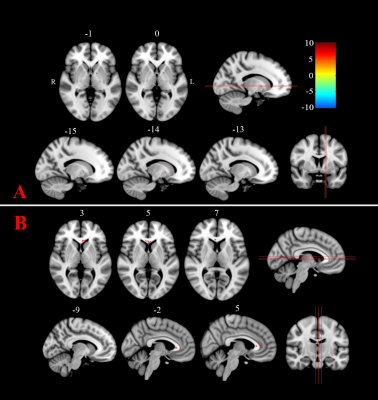
The ablated point could be seen clearly on the MP2RAGE image obtained 1 month postoperatively (Figure 2). The average TDI value in the ablated point was significantly lower after surgical treatment (3010.3±391.3) compared with before (3751.3±755.8). For the FA values, no significant difference was found between groups (P=0.073 after family-wise error [FWE] correction). However, TDI values were found significantly reduced (P=0.001 with FWE correction, cluster>10) after surgical treatments compared with before in the brain areas of the genu of corpus callosum (CC; 3177.7±572.7 vs. 3554.8±567.6) and left globus pallidus (1614.4±265.0 vs. 2004.7±198.6), as shown in Figure 3.Discussion
Understanding the microstructural change in patients with PD after MRgFUS can help guide treatment. FA values, estimated from the relatively low spatial resolutions and noisy diffusion images, might neglect microanatomic changes owing to the partial volume effects, as demonstrated in this study. TDI provides high-quality images with high spatial resolutions and sharp anatomic contrast to detect the microstructural changes that overcome low-resolution FA defects. The genu of CC is a large white-matter tract that projects fibers to connect medial and lateral surfaces of the frontal lobes. Decreased TDI values in the genu of CC might be related to the relief of tremor symptoms in patients with PD after MRgFUS therapy. The left globus pallidus has been reported to be responsible for PD tremors [6] and is close to the DRTC pathway. Therefore, tremor relief could reduce tract densities in this important subcortical nucleus.Conclusion
TDI can detect microstructural changes induced by MRgFUS therapy in the brains of patients with tremor-dominant PD that could help radiologists and health care providers determine the best course of treatment for these patients.Acknowledgements
National Key R&D Program of China (2017YFE0103600), National Natural Science Foundation of China (81720108021), Zhongyuan Thousand Talents Plan Project(ZYQR201810117), Zhengzhou Collaborative Innovation Major Project (20XTZX05015)References
[1] Zur G , Lesman-Segev O H , Schlesinger I , et al. Tremor Relief and Structural Integrity after MRI-guided Focused US Thalamotomy in Tremor Disorders[J]. Radiology, 2020, 294(3):191624.
[2] Calamante, F., Tournier, J.-D., Jackson, G.D., Connelly, A., 2010. Track Density Imaging(TDI): super resolution white matter imaging using whole-brain track densitymapping. Neuroimage 53, 1233–1243.
[3] Barajas R F , Hess C P , Phillips J J , et al. Super-Resolution Track Density Imaging of Glioblastoma: Histopathologic Correlation[J]. American Journal of Neuroradiology, 2013, 34(7):1319-1325.
[4] Calamante F , Tournier J D , Heidemann R M , et al. Track density imaging (TDI): Validation of super resolution property[J]. NeuroImage, 2011, 56(3):1259-1266.
[5] Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54(3):2033–2044.
[6] Lang A E , Lozano A M . Parkinson's Disease[J]. Lancet, 1998, 386(9996):896-912.
Figures