0542
Microstructure of grey matter nuclei in early Parkinson’s disease: longitudinal study using diffusion kurtosis imaging1National Neuroscience Institute, Singapore, Singapore, 2Duke-NUS Medical School, Singapore, Singapore, 3Singapore General Hospital, Singapore, Singapore
Synopsis
We show that the diffusion kurtosis characteristics of grey matter nuclei in early Parkinson's disease were abnormal and that this abnormality was maintained over two years. Furthermore, elevated mean kurtosis was associated with worsening motor function. This supports the use of diffusion kurtosis imaging to characterise tissue microstructure and potentially monitor disease progression even in early Parkinson's disease.
Introduction
Diffusion kurtosis imaging (DKI) may act as a direct, quantitative, and objective measure of tissue microstructure to aid in Parkinson’s disease (PD) diagnosis and monitoring [1]. However, there are no longitudinal studies of early PD using DKI.We aimed to determine whether DKI characteristics of key PD-related nuclei would be altered between baseline and two-year timepoints in early PD compared to controls and correlate with the change in motor symptoms.
Methods
Early PD and healthy control subjects were recruited from two tertiary hospitals in Singapore for a large longitudinal study including MRI and clinical assessment at baseline and two years. MRI was performed on a 3T Siemens Skyra, and included DKI (TE=0.102s, TR=10.118s, FA=90°, voxel size=1.8x1.8x2.5mm, matrix=112x112x55, 60 directions, b=1000,2000mm/s2) and T1-weighted MPRAGE (TE=0.002s, TR=1.900s, FA=9°, voxel size=1.0x1.0x1.0mm, matrix=256x256x256) scans.Clinical assessment during the same visit included the Movement Disorders Society Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS-3 [2]), Hoehn & Yahr stage (H&Y [3]) and Montreal Cognitive Assessment (MOCA [4]).
Diffusion data were processed using MRTrix3 with the following steps: denoising, correction of Gibbs’ ringing artefacts, motion and eddy current correction (using FSL eddy [5]), bias field correction (using ANTS [6]) and within-subject intensity normalisation to the median CSF value. DKI parameters were then calculated using Diffusion Kurtosis Estimator to produce maps of the following metrics: axial, radial and mean diffusivity (AD, RD, MD), fractional anisotropy (FA), axial radial and mean kurtosis (AK, RK, MK), and fractional anisotropy of kurtosis (KFA).
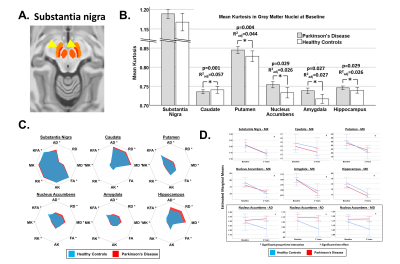
Masks for six key PD-related grey matter regions of interest (ROIs; substantia nigra [SN], putamen, caudate, nucleus accumbens, hippocampus and amygdala; Figure 1a) were extracted from FreeSurfer [7] and CIT168 atlases [8] and registered to individuals’ diffusion data by affine, then non-linear registration using FLIRT/FNIRT [9]. In each ROI, we extracted the mean value of each DKI metric.
We used repeated-measures ANOVA to test the interaction of group and time for each region, focusing on the MK as the primary imaging measure and other metrics as secondary. We also tested the Pearson correlation between imaging metrics (at baseline and change over two years) and the UPDRS-MDS-3.
Results
262 participants were included in the study. The PD group (n=185; aged 67.5±9.1 years; 43% female; MDS-UPDRS-3 20.8±9.8; H&Y 1.7±0.4; disease duration 135.5±100.8 days) and matched healthy control group (n=77; aged 66.6±8.1 years; 53% female) had an average interval of 2.03±0.24 years between baseline and follow-up.At baseline, MK was higher in PD than controls in the putamen, nucleus accumbens, amygdala and hippocampus when adjusting for age, sex and education but not the SN (R2adj>0.026, p<0.029; Figure 1b). In the secondary DKI metrics, the PD group had predominantly increased diffusivity, increased kurtosis, lower KFA and no differences in FA across regions (Figure 1c). The SN had relatively high kurtosis compared to other ROIs, and the greatest differences between groups were found in the diffusivity of the hippocampus.
In the longitudinal tests, we found no significant group-time interactions for MK in any region but, for the secondary DKI indices, there were significant group-time interactions for axial, radial and mean diffusivity (p=0.036, 0.032, 0.029, respectively), where diffusivity fell in controls at the expected rate due to ageing but remained static in PD (figure 1d, black box).
The two-year change in MK in the amygdala was significantly negatively correlated with the corresponding change in motor performance (r=-0.25, p=0.018).
Worsening of the motor performance was positively correlated with the baseline diffusivity and kurtosis fractional anisotropy (r>0.24, p<0.024).
Discussion
We present the first longitudinal study to use DKI in a large cohort of early PD patients. Our findings show that MK was increased in PD, possibly by the structural break-down of cells and resulting increased geometric complexity of the cellular environment [1]. In agreement with previous studies [10, 11], we found elevated MK in specific basal ganglia and limbic regions, which was maintained over two years and significantly correlated with worsening motor function in PD. Kurtosis was differentially altered over two years between PD and controls in diffusivity of the nucleus accumbens, which is a key site for motor-limbic integration in PD. Baseline diffusivity and KFA of the putamen were the best candidates to predict clinical worsening. Together, this work supports the idea of DKI as a potentially useful future marker of PD pathology.Conclusion
DKI imaging markers can be used to characterise tissue microstructure and potentially to monitor PD progression.Acknowledgements
No acknowledgement found.References
1. Jensen, J.H., et al., Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. magnetic resonance in medicine, 2005. 53(6): p. 1432-1440.
2. Goetz, C.G., et al., Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. 2008. 23(15): p. 2129-2170.
3. Goetz, C.G., et al., Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson's disease. 2004. 19(9): p. 1020-1028.
4. Nasreddine, Z.S., et al., The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. 2005. 53(4): p. 695-699.
5. Andersson, J.L.R. and S.N. Sotiropoulos, An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 2016. 125: p. 1063-1078.
6. Avants, B.B., N. Tustison, and G.J.I.j. Song, Advanced normalization tools (ANTS). 2009. 2(365): p. 1-35.
7. Fischl, B., FreeSurfer. NeuroImage, 2012. 62(2): p. 774-781.
8. Pauli, W.M., A.N. Nili, and J.M. Tyszka, A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Scientific Data, 2018. 5(1): p. 180063.
9. Andersson, J.L., M. Jenkinson, and S.J.F.A.G.o.t.U.o.O. Smith, Non-linear registration aka Spatial normalisation FMRIB Technial Report TR07JA2. 2007: p. 1-22.
10. Surova, Y., et al., Alteration of putaminal fractional anisotropy in Parkinson's disease: a longitudinal diffusion kurtosis imaging study. Neuroradiology, 2018. 60(3): p. 247-254.
11. Wang, J.J., et al., Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology, 2011. 261(1): p. 210-7.
Figures