0538
Peritumoral Dilation Radiomics of Gd-EOB-DTPA MRI Predicts Early Relapse in Hepatocellular Carcinoma Without Macrovascular Invasion1Shanghai Institute of Medical Imaging, Shanghai, China, 2Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China, 3Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China, 4Shanghai United Imaging Intelligence Co., Ltd., Shanghai, China, 5Department of Medical Imaging, Shanghai Medical College, Fudan University, Shanghai, China
Synopsis
Preoperatively identifying predisposing predictors of early relapse is crucial for stratifying patient risk, performing prompt intervention and improving long-term outcome. Whether peritumoral dilation radiomics can predict early recurrence in hepatocellular carcinoma(HCC) patients without macrovascular invasion remains unclear. Hence, we developed a bi-regional (the entire tumor and peritumoral zone within 1 cm) radiomics of gadoxetate disodium-enhanced (Gd-EOB-DTPA) MRI, which derived a satisfying discrimination in 2-year recurrence by the 5-fold cross-validation method.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent malignancies and the third leading cause of cancer-related death globally 1. Given that the 5-year recurrence rate reaches 50%-70% after liver resection 2, the major obstacle to improving long-term survival is postoperative recrudescence 3. Pathologically, peritumoral parenchyma is representative of cancerous heterogeneity and rich in highly invasive cells 4, as well as vulnerable to microvascular invasive (MVI) 5,6 and satellite nodules 4. Consequently, peritumoral zone is more susceptible to intrahepatic metastasis and dismal outcome. Hitherto, few radiomics studies 7,8 have focused on this highly aggressive region for prognostic analyses. Although gadoxetate disodium-enhanced (Gd-EOB-DTPA) MRI exerts crucial impacts on the management of HCC patients, it provides limited quantitative hallmarks than multiparameter radiomics 9. Radiomics, a novel and non-invasive imaging tool, can extract high-throughput and high-dimensional signatures from multi-modality imaging to improve diagnostic or prognostic accuracy, benefit the characterization of lesions, and heighten the surveillance management of patients 9,10. Therefore, this study intended to construct a preoperatively bi-regional radiomics of Gd-EOB-DTPA MRI for predicting 2-year recurrence in HCC patients without macrovascular invasion.Methods
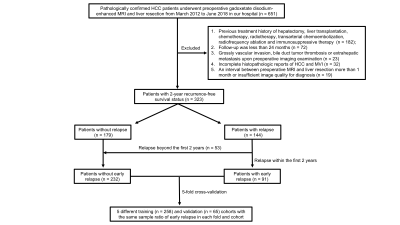
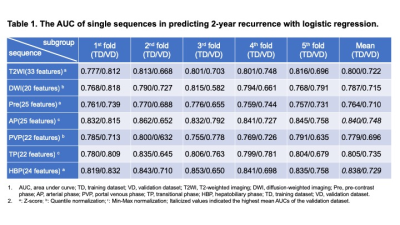
Between March 2012 and June 2018, 323 pathologically confirmed HCC patients who preoperatively underwent Gd-EOB-DTPA MRI were recruited. Uni- and multivariate logistic regressions identified independent predictors for early (≤2 years) recrudescence. The bi-regional radiomics features were extracted from 7-sequence images, verified by 5-fold cross-validation, and thus derived an average performance. Independently clinicoradiologic predictors were incorporating with radiomics model for developing a comprehensive model. The predictive discrimination was quantified with the area under curve (AUC) of the receiver operating characteristic curve.Results
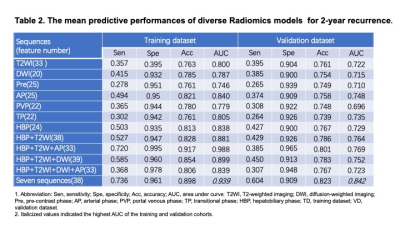
With the median time of recurrence-free survival (RFS) reaching 60.43 months, 28.2% (91/323) and 16.4% (53/323) patients suffered from early and delay relapse, respectively. In the clinicoradiologic model, histologic MVI (OR 2.132, 95%CI 1.122-4.050, P = 0.021), tumor size >5 cm (OR 3.661, 95%CI 1.094-12.25, P = 0.035), alanine aminotransferase >50 U/L (ALT; OR 2.173, 95%CI 1.066-4.430, P = 0.033), γ-glutamyl transpeptidase >60 U/L (GGT; OR 1.941, 95%CI 1.062-3.548, P = 0.031), prealbumin ≤240 mg/L (OR 2.078, 95%CI 1.021-4.231, P = 0.044) and peritumoral enhancement (OR 1.826, 95%CI 0.989-3.374, P = 0.054) independently impaired 2-year RFS. Nevertheless, these indicators only yielded a moderate predictive performance for 2-year relapse (sensitivity 0.615; specificity 0.711; AUC: 0.694, 95%CI: 0.628-0.760, P<0.001), and were paucity of robustness (P > 0.05) when integrating with the peritumoral dilation radiomics. Based on the 38 bi-regional features extracted from 7 sequences, our radiomics model — the ultimate predictive model — obtained mean AUCs of 0.939 (95% CI 0.908-0.973) in the training dataset and 0.842 (95% CI 0.736-0.951) in the validation cohort. Notably, four of six top discriminating signatures involved the homogeneity of bi-region, implying “ the greater heterogeneity, the more 2-year recurrence”.Discussion
Previous studies have reported that MVI 11-13, peritumoral enhancement 14, tumor size beyond 5 cm 3,13,15, decreased prealbumin value 16, elevated ALT 17,18and GGT 19-21values shorten the RFS independently, conforming with our findings in the clinicoradiologic model. Nonetheless, above indexes failed to independently predict 2-year RFS when incorporating with the bi-regional radiomics model. Meanwhile, our peritumoral dilation radiomics also outperformed the clinicoradiologic model for 2-year recurrence after curative resection of An et al study 14. The reason may that the robust bi-regional algorithm has merged high-dimensional signatures of peritumoral enhancement and histologic MVI located at peritumoral areas within 1 cm — high-hazard spreading scopes of micro-metastasis (MVI and satellite nodules) 4,22. Besides, four of the six most relapse-related signatures are highly consistent with the well-known cognition that "the greater heterogeneity, the more aggressive behavior, treatment-resistant and dismal prognosis 23”. Hence, we suppose that the HCC patients with high-risk recurrence by our non-invasive bi-regional radiomics can 1) properly enlarge resection margin or even adopt anatomical hepatectomy; 2) choose neoadjuvant therapy before surgery; 3) adhere to normative follow-up strategy.Conclusions
As an independently robust and reliable biomarker of early recurrence, the peritumoral dilation radiomics facilitates the non-invasive burgeon of individualized treatment and surveillance regimens.Acknowledgements
Funding: Supported by National Natural Science Foundation of China (No.91859107), Shanghai Science and Technology Committee (No.19411965500), Shanghai Municipal Key Clinical Specialty (No.W2019-018), Clinical Research Plan of SHDC (No.SHDC2020CR1029B).References
1. Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet (London, England)391, 1301-1314 (2018).
2. Vogel, A., et al.Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology 29, 238-255 (2018).
3. Wang, M.D., et al.Early and Late Recurrence of Hepatitis B Virus-Associated Hepatocellular Carcinoma. Oncologist(2020).
4. Cong, W.M., et al.Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol22, 9279-9287 (2016).
5. Hu, H.T., et al.Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY)43, 3324-3330 (2018).
6. Hu, H., et al.A non-smooth tumor margin on preoperative imaging assesses microvascular invasion of hepatocellular carcinoma: A systematic review and meta-analysis. Sci Rep7, 15375 (2017).
7. Song, W., et al.MRI-Based Radiomics: Associations With the Recurrence-Free Survival of Patients With Hepatocellular Carcinoma Treated With Conventional Transcatheter Arterial Chemoembolization. J Magn Reson Imaging52, 461-473 (2020).
8. Shan, Q.Y., et al.CT-based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging19, 11 (2019).9. Wei, J., et al.Radiomics in liver diseases: Current progress and future opportunities. Liver Int(2020).
10. Lambin, P., et al.Radiomics: the bridge between medical imaging and personalized medicine. Nature reviews. Clinical oncology14, 749-762 (2017).
11. Xu, X., et al.Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol70, 1133-1144 (2019).
12. Shindoh, J., et al.Microvascular Invasion and a Size Cutoff Value of 2 cm Predict Long-Term Oncological Outcome in Multiple Hepatocellular Carcinoma: Reappraisal of the American Joint Committee on Cancer Staging System and Validation Using the Surveillance, Epidemiology, and End-Results Database. Liver Cancer9, 156-166 (2020).
13. Hwang, S., et al.The Impact of Tumor Size on Long-Term Survival Outcomes After Resection of Solitary Hepatocellular Carcinoma: Single-Institution Experience with 2558 Patients. J Gastrointest Surg19, 1281-1290 (2015).
14. An, C., et al.Single Hepatocellular Carcinoma: Preoperative MR Imaging to Predict Early Recurrence after Curative Resection. Radiology276, 433-443 (2015).
15. Miltiadous, O., et al.Progenitor cell markers predict outcome of patients with hepatocellular carcinoma beyond Milan criteria undergoing liver transplantation. J Hepatol63, 1368-1377 (2015).
16. Li, J.D., et al.Preoperative prealbumin level as an independent predictor of long-term prognosis after liver resection for hepatocellular carcinoma: a multi-institutional study. HPB (Oxford)21, 157-166 (2019).
17. Iwadou, S., et al.Time-dependent analysis of predisposing factors for the recurrence of hepatocellular carcinoma. Liver Int30, 1027-1032 (2010).18. Maeda, T., et al.Prognosis of early hepatocellular carcinoma after hepatic resection. Hepato-gastroenterology55, 1428-1432 (2008).
19. Wu, S.J., et al.Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg36, 143-151 (2016).
20. He, L.L., et al.Independent risk factors for disease recurrence after surgery in patients with hepatitis B virus-related hepatocellular carcinoma ≤3 cm in diameter. Gastroenterology report7, 250-257 (2019).
21. Song, P., et al.High Levels of Gamma-Glutamyl Transferase and Indocyanine Green Retention Rate at 15 min as Preoperative Predictors of Tumor Recurrence in Patients With Hepatocellular Carcinoma. Medicine (Baltimore)94, e810 (2015).
22. Shi, M., et al.[Micrometastasis distribution in liver tissue surrounding hepatocellular carcinoma]. Zhonghua zhong liu za zhi [Chinese journal of oncology]24, 257-260 (2002).
23. Lubner, M.G., Smith, A.D., Sandrasegaran, K., Sahani, D.V. & Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics : a review publication of the Radiological Society of North America, Inc37, 1483-1503 (2017).