0537
Automatic Detection of Small Hepatocellular Carcinoma (≤2 cm) in Cirrhotic Liver based on Pattern Matching and Deep Learning1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, Shanghai Chest Hospital, Shanghai, China, 3Human Phenome Institute, Fudan University, Shanghai, China, 4Market Solutions Center, Philips Healthcare, Shanghai, China, 5Department of Radiology, Ruijin Hospital, Shanghai, China
Synopsis
This study presented an algorithm for small hepatocellular carcinoma (sHCC) detection and segmentation in cirrhotic liver based on diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) images. The model included two-steps: screening of suspicious lesions in DWI using pattern matching algorithm; identification and segmentation of true lesions in DCE based on deep learning. The proposed model exhibited superior performance in sHCC (≤2 cm) detection and segmentation, which significantly outperformed the Liver Imaging Reporting and Data System (LI-RADS) based diagnosis.
INTRODUCTION
Early detection of hepatocellular carcinoma (HCC) is crucial for clinical management. The hallmark imaging characteristics of HCC include arterial phase hyperenhancement, followed by a washout on portal venous and/or delayed phase 1,2. However, sHCC ≤2 cm in size predisposes to exhibit atypical imaging characteristics, resulting in low diagnostic sensitivity 3-8. Besides, accurate imaging interpretation is time-consuming and challenging for radiologists. Current studies have reported large HCC detections using automatic algorithms, but there is a lack of research on automatic detection of sHCCs.METHODS
Data: A retrospective study included 4480 images from 56 cirrhosis patients (28 sHCC patients with 32 pathologically confirmed lesions and 28 non-HCC cirrhosis patients) were included to build and validate the model through five-fold cross-validation. An external test cohort including 2400 images from 30 cirrhosis patients (15 sHCC patients with 18 lesions and 15 non-HCC cirrhosis patients) was included to further verify the generalization capability of the proposed model.MR imaging: The MRI protocols included free-breathing DWI with b values of 0 and 800 mm2/s, and gadopentetate dimeglumine enhanced DCE imaging with a fat-suppressed three-dimensional (3D) T1-weighted gradient-echo (GRE) sequence with echo time (TE) of 1.32 ms, repetition time (TR) of 3.70 ms, in-plane resolution of 1.6×1.6 mm2, and slice thickness of 4.0 mm.
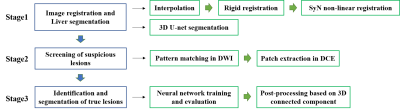
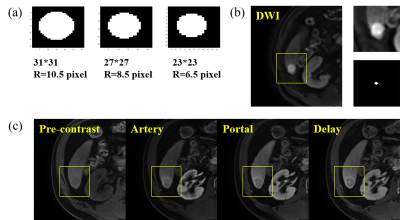
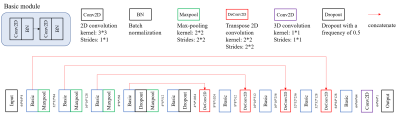
PM-DL model: The proposed pattern matching and deep learning (PM-DL) model consisted of three main steps (Figure 1): a) 3D co-registration between DWI and DCE images using symmetric normalization (SyN) algorithm and liver segmentation based on a 3D U-net; b) screening of suspicious lesions on DWI images based on pattern matching algorithm with three circular templates of different scales, the center of the eligible area was recorded for the extraction of image patches in the corresponding DCE images (Figure 2); c) identification/segmentation of sHCC lesions on DCE images with a modified U-net (Figure 3).
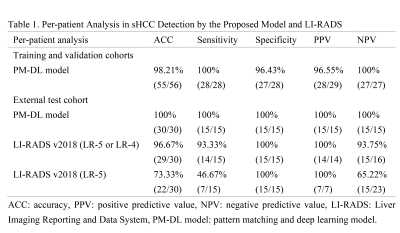
Evaluation metrics: The performance of lesion detection was assessed by per-lesion and per-patient analysis through quality metrics including accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The segmentation performance was evaluated using the DICE coefficient. The lesion volumes and largest sizes were compared between manually delineated lesions by experienced radiologists and model predicted ones.
RESULTS
The proposed PM-DL model achieved a sensitivity of 100% (32/32) and PPV of 86.49% (32/37) for per-lesion analysis, and a sensitivity of 100% (28/28) and specificity of 96.43% (27/28) for per-patient analysis in the validation cohort. No significant difference was found between manually delineated lesions and model predicted ones in measurements of volume (2.66 ± 1.02 vs. 2.35 ± 1.28 mm3, P = 0.19) and largest size (1.72 ± 0.19 vs. 1.69 ± 0.22 mm2, P = 0.58). The DICE coefficient was 0.74 ± 0.15.Similar performances were identified in the external test cohort with a sensitivity of 88.89% (16/18) and a positive predictive value of 80.00% (16/20) in the external test cohort for per-lesion analysis, and a sensitivity of 100% (15/15), and specificity of 100% (15/15) for per-patient analysis. No significant difference was found between manually delineated lesions and PM-DL model predicted lesions for volume (2.47 ± 1.09 vs. 1.98 ± 1.28 mm3, P = 0.10) and largest size (1.72 ± 0.24 vs. 1.67 ± 0.25 mm2, P = 0.42) measurements. The DICE coefficient was 0.77 ± 0.10.
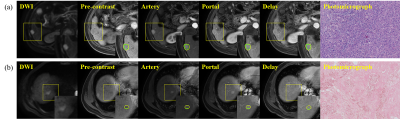
Moreover, the PM-DL model outperformed Liver Imaging Reporting and Data System (LI-RADS) in diagnostic sensitivity (probable HCCs: LR-5 or LR-4, P = 0.32; definite HCCs: LR-5, P < 0.01), with a comparable specificity (Table 1). Two representative cases of sHCC detected by the PM-DL model was shown in Figure 4, one of which can be determined by LI-RADS as probable HCC (LR-4, Figure 4(a)), while the other was scored as LR-M (Figure 4(b)).
DISCUSSION
Due to the wide existence of overlapping image features between sHCCs and benign cirrhosis-associated nodules, automatic detection of sHCC in cirrhotic liver is extremely challenging. It is difficult to apply deep learning algorithms directly on the DCE images for sHCC detection due to the small sizes of lesions and the interference from nodules. Herein, we proposed a PM-DL model for automatic detection of sHCC from the cirrhotic liver through a two-step algorithm. The accurate detection of sHCCs from the cirrhotic liver background has confirmed that the proposed PM-DL model could distinguish the malignant nodules from precursor nodules. Moreover, the results demonstrate the proposed model yields increased sensitivity in comparison to LI-RADS v2018.CONCLUSION
This study implemented pattern matching and deep learning algorithms to detect and segment sHCCs (≤2 cm) in the cirrhotic liver. The superior performance both in the validation cohort and external test cohort indicated the proposed PM-DL model may be feasible for automatic detection of sHCCs with high accuracy, which can greatly help doctors in clinical diagnosis and treatment.Acknowledgements
No acknowledgement found.References
1. Choi JY, Lee JM and Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272(3):635-654.
2. Choi JY, Lee JM and Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273(1):30-50.
3. Kierans AS, Kang SK and Rosenkrantz AB. The Diagnostic Performance of Dynamic Contrast-enhanced MR Imaging for Detection of Small Hepatocellular Carcinoma Measuring Up to 2 cm: A Meta-Analysis. Radiology. 2016;278(1):82-94.
4. Yu JS, Chung JJ, Kim JH, et al. Small hypervascular hepatocellular carcinomas: value of “washout” on gadolinium-enhanced dynamic MR imaging compared to superparamagnetic iron oxide-enhanced imaging. Eur. Radiol. 2009;19(11):2614-2622.
5. Kim TK, Lee KH, Jang HJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1-2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011;259(3):730-738.
6. Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264(3):761-770.
7. Rhee H, Kim MJ, Park YN, et al. Gadoxetic acid-enhanced MRI findings of early hepatocellular carcinoma as defined by new histologic criteria. J. Magn. Reson. Imaging. 2012;35(2):393-398.
8. Park VY, Choi JY, Chung YE, et al. Dynamic enhancement pattern of HCC smaller than 3 cm in diameter on gadoxetic acid-enhanced MRI: comparison with multiphasic MDCT. Liver Int. 2014;34(10):1593-1602.
Figures