0535
Utility of Texture Analysis on Quantitative Susceptibility Maps to Stage Hepatic Fibrosis1Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, East China Normal University, shanghai, China, 2Department of Radiology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, shanghai, China, 3Department of Liver Diseases, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, shanghai, China, 4MR Collaboration NE Asia, Siemens Healthcare, shanghai, China, 5Department of Radiology, Weill Medical College of Cornell University, New York, NY, United States
Synopsis
Hepatic fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) proteins including collagen, which would contribute strong diamagnetic susceptibility in the liver tissue due to enhanced density of orbiting electrons. This study measured the texture features on susceptibility maps in patients with chronic liver diseases. The results showed that some of the second-order texture parameters were significantly different between cohorts of significant hepatic fibrosis (Ishak-F ≥ 3) and non-significant hepatic fibrosis (Ishak-F < 3). The texture analysis on susceptibility maps may have the potential to stage hepatic fibrosis.
Purpose
The objective of this study was to evaluate the feasibility of texture analysis on liver susceptibility maps in differentiating cohorts of significant hepatic fibrosis (Ishak-F ≥ 3) and non-significant hepatic fibrosis (Ishak-F < 3).Materials and Methods
There were 30 consecutive patients with chronic liver diseases (CLDs) who underwent liver biopsy and MRI-QSM examination in this study.The patients were imaged on a clinical 3T MRI system (Skyra; Siemens Healthcare, Erlangen, Germany) using an 18-channel body coil in combination with some elements of the spine coil. To obtain the susceptibility maps, complex data were acquired by using a 3D monopolar readout gradient volumetric interpolated breath-hold examination (VIBE) sequence with the following parameters: TR = 11.3 ms, flip angle = 4°, echo number = 6, TE1 = 1.07 ms, ΔTE = 1.79 ms, bandwidth = 1060 Hz/pixel, FOV = 400×350 mm2, matrix size = 224×196×52, voxel size = 1.8×1.8×3.5 mm3. Additionally, a CAIPIRINHA parallel imaging with acceleration factor of 2×2 and 6/8 fractional Fourier transform in the phase encoding direction were used to reduce acquisition time. The acquisition time for each sequence was 17 seconds during one breath-hold.Simultaneous phase unwrapping and removal of chemical shift (SPURS)1 using graph cuts with conditional jump moves were first performed on the phase images, followed by fine-tuning of the field map with T2*-IDEAL with a single R2* and 6-peak fat model for combined water-fat signal. The output inhomogeneity B0 field of T2*-IDEAL was then processed with background field removal using the projection-onto-dipole-field (PDF) method2, and the remaining magnetic field was processed to generate a susceptibility map using the morphology enabled dipole inversion algorithm (MEDI)3.
A region of interest (ROI) covering the liver was manually drawn in the slice in the anterior and posterior segment of the right lobe of the liver where a biopsy specimen was obtained. The first- and second- order texture analyses of the segmented liver were performed using MaZda software (http://www.eletel.p.lodz.pl/programy/mazda/, Lodz, Poland)4. The first-order texture parameters included mean, variance, skewness and kurtosis. The second-order texture parameters included angular second moment (AngScMom), contrast, correlation, difference of variance (DifVarnc), entropy, inverse different moment, sum of entropy, sum of average (SumAverg), sum of variance (SumVarnc), difference of entropy (DifEntrp), and sum of squares (SumOfSqs).
Differences in first- and second- order texture parameters of QSM images between cohorts of non-significant and significant fibrosis were compared using Mann-Whitney U test. Furthermore, receiver operating characteristic (ROC) curves were further used for assessing the ability of the statistically significant texture features to distinguish cohorts of significant hepatic fibrosis from non-significant hepatic fibrosis. SPSS statistical software was used to conduct all statistical analyses.
RESULTS
Four patients with incomplete results from biopsy or histopathologic diagnosis were excluded from further analysis. The final cohort consisted of 26 patients (mean age, 48.08 ± 12.03 years; range, 27-68 years).There were no significant differences in the first-order texture parameters of QSM images between two cohorts of hepatic fibrosis. There were significant differences between two cohorts in DifVarnc (P=0.002), entropy (P = 0.002), contrast (P=0.002), DifEntrp (P=0.013), AngScMom (P=0.020) and InvDfMom (P=0.047). Fig 1. shows the results of the second-order texture analyses for the QSM maps.
The results of the ROC curve analyses of the QSM texture parameters between cohorts of significant and non-significant fibrosis are summarized in Table 1. The DifVarnc of susceptibility maps yielded the optimal performance for differentiating significant from non-significant hepatic fibrosis (AUC = 0.848, P=0.002), The optimal cutoff value of DifVarnc for significant versus non-significant hepatic fibrosis was > 205.96 with Youden index of 0.642.
Discussion and conclusion
This was the first study to evaluate the texture features of susceptibility maps in hepatic fibrosis. The QSM texture analysis successfully distinguished the cohorts of significant and non-significant fibrosis. The AUC for DifVarnc, entropy and contrast were 0.848, 0.842, and 0.842, respectively, demonstrating that texture analysis of susceptibility maps obtained higher AUC values in staging hepatic fibrosis than the texture features of unenhanced T15,T1-weighted6, T2-weighted7 and diffusion-weighted imaging8.In conclusion, texture analysis on susceptibility maps has the potential to discriminate between the cohorts of significant and non-significant hepatic fibrosis.
Acknowledgements
No acknowledgement found.References
1. Dong J, Liu T, Chen F, et al. Simultaneous phase unwrapping and removal of chemical shift (SPURS) using graph cuts: application in quantitative susceptibility mapping. IEEE Trans Med Imaging 2015;34:531-540.
2. Liu T, Khalidov I, de Rochefort L, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed 2011;24:1129-1136.
3. Liu J, Liu T, de Rochefort L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012;59:2560-2568.
4. Szczypinski PM, Strzelecki M, Materka A, Klepaczko A. MaZda--a software package for image texture analysis. Comput Methods Programs Biomed 2009;94:66-76.
5. Xu X, Zhu H, Li R, et al. Whole-liver histogram and texture analysis on T1 maps improves the risk stratification of advanced fibrosis in NAFLD. Eur Radiol 2020.
6. Cannella R, Borhani AA, Tublin M, Behari J, Furlan A. Diagnostic value of MR-based texture analysis for the assessment of hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). Abdom Radiol (NY) 2019;44:1816-1824.
7. Mayerhoefer ME, Schima W, Trattnig S, Pinker K, Berger-Kulemann V, Ba-Ssalamah A. Texture-based classification of focal liver lesions on MRI at 3.0 Tesla: a feasibility study in cysts and hemangiomas. J Magn Reson Imaging 2010;32:352-359.
8. Zheng Y, Xu YS, Liu Z, et al. Whole-Liver Apparent Diffusion Coefficient Histogram Analysis for the Diagnosis and Staging of Liver Fibrosis. J Magn Reson Imaging 2020;51:1745-1754.
Figures
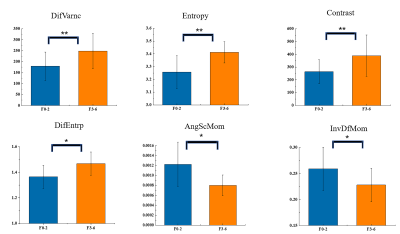

Table 1. The results of the ROC analyses of the texture features extracted from QSM between cohorts of significant and non-significant hepatic fibrosis patients.
Only the results with statistically significant differences between the cohorts of significant and non-significant hepatic fibrosis are shown. AUC = area under receiver operating characteristic curve, SS = sensitivity, SP = specificity. DifVarnc = difference of variance, DifEntrp = difference of entropy, AngScMom = angular second moment, InvDfMom = inverse different moment.