0534
Deep Learning-based Adaptive Image Combination for Signal-Dropout Suppression in Liver DWI1Pattern Recognition Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Signal-dropouts due to pulsation are one of the most prominent artifacts in diffusion-weighted imaging (DWI) of the liver. It can affect a significant portion of the repetitions acquired for a given slice. Instead of performing uniform averaging which might result in locally attenuated liver signal, this work proposes to train a convolutional neural network (CNN) to estimate smooth weight maps for individual repetitions. This allows to locally suppress signal-dropouts, resulting in more homogeneous liver signal while maintaining signal-to-noise ratio (SNR) in artifact-free image regions.
Introduction
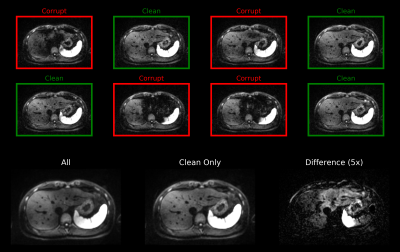
Given its high sensitivity for lesion detection [1,2], DWI is an integral part of many standard clinical protocols for liver MRI. Despite the frequent use of DWI, however, image quality is still variable which can lead to false diagnoses and costly follow-up examinations. One class of frequently observed artifacts are signal-dropouts caused by pulsation. Due to its proximity to the heart, the left liver lobe is particularly prone to this kind of artifact. In a typical setting, in which several repetitions of the same slice are acquired, up to 70% of the repetitions can be affected by signal-dropouts. Consequently, the averaged image might exhibit inhomogeneous liver signal (Figure 1) which results in biased ADC maps further down-stream.Previous work proposed to reduce artifacts by either applying motion-robust diffusion preparation schemes [3,4] or dedicated post-processing [5,6]. For example, in the CNN-based approach presented in [6], images affected by signal-dropouts were detected and discarded before averaging. However, since signal voids typically only affect limited parts of the image, discarding entire repetitions reduces SNR efficiency.
In this work, we propose to learn smooth and spatially varying combination weights for the single repetitions using a CNN. Given a set of repetitions as input, the network produces corresponding weight maps which allow local suppression of signal-dropouts without sacrificing SNR in artifact-free image regions.
Methods
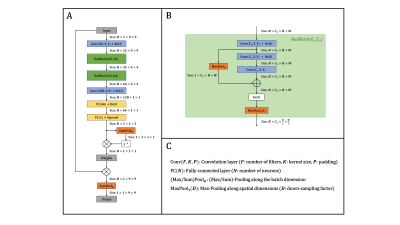
Data: Using a prototypical single-shot EPI sequence, free-breathing liver DWI (b-values: 50 and 800 s/mm2) was acquired in 25 volunteers on 1.5 and 3 T MR scanners (MAGNETOM, Siemens Healthcare, Erlangen, Germany). While 19 and two volunteer data sets served as training and validation splits, respectively, the remaining four were used for evaluation purposes. The individual repetitions of all acquired slices were screened for signal-dropouts and were manually labeled as either ‘clean’ or ‘corrupt’. Given $$$N$$$ repetitions out of which $$$M$$$ were considered clean, the training ground-truth was generated by averaging the $$$M$$$ clean repetitions, while the input of the network consisted of $$$M$$$ randomly selected repetitions.Network: Given a set of patches (9$$$\times$$$9 pixels), the proposed network produces a normalized scalar for every patch which is then used to compute a weighted sum (Figure 2). During inference, whole images can be processed using a sliding window where results at overlapping locations are averaged before normalization. Two central requirements needed to be fulfilled by the network: 1) permutation-equivariance (i.e., the output should be independent of the ordering of the input elements) and 2) the ability to handle different set sizes. To achieve this, the concept of Deep Sets [7] was employed by processing every patch individually ‒ e.g. by treating the set as a typical input batch ‒ but additionally including pooling operations along the batch dimension to provide the network with set statistics.
Training: During training, corresponding patches were randomly cropped from the input images and the ground-truth. The training objective was to maximize the structural similarity (SSIM) between the output and the ground-truth patches. All parameters were optimized using Adam [8] with a learning rate of 10-4 until SSIM across the validation set did not improve over 30 epochs. The batch size varied across forward passes as it depended on the number of repetitions of a given slice.
Evaluation: The CNN-based adaptive image combination was assessed qualitatively on both DW images as well as the derived ADC maps. For the latter, quantitative evaluation was additionally performed by analyzing ADC values in regions-of-interests (ROIs) that were placed into the left liver lobe for 40 slices from the test data set. Results were compared against uniform averaging for different percentages of corrupt repetitions.
Results & Discussion
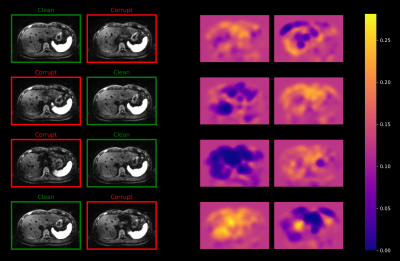
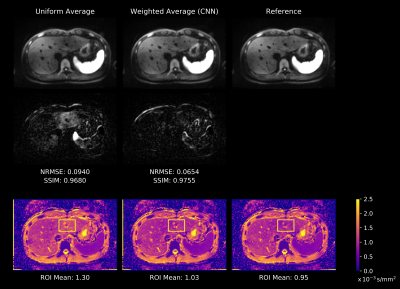
Figure 3 shows weight maps produced by the network for a given set of repetitions. As illustrated, locations with lower weight approximately correspond to locations that are affected by signal-dropouts. This implies that the network is able to locally suppress affected regions, while the remaining parts of the images contribute with approximately uniform weight.Figure 4 demonstrates that uniform averaging leads to an attenuated left liver lobe in the DW image and consequently to an overestimation of the ADC. In contrast, a weighted sum using the maps from Figure 3 homogenizes liver signal in the DW image. This results in more reliable ADC values which are in agreement with the reference image obtained from uniform averaging of artifact-free repetitions.
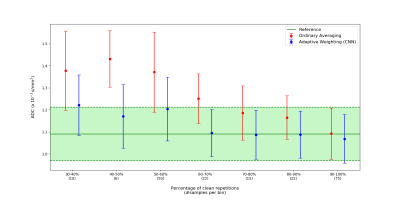
The quantitative ROI analysis presented in Figure 5 shows how ADC values in the left liver lobe are corrected when comparing the proposed adaptive combination to uniform averaging. Even if up to 70% of the repetitions are corrupt, the network is still able to recover DW signal and hence reduce the ADC significantly.
Conclusion
This work demonstrates that estimating smooth weight maps using a CNN efficiently addresses the problem of signal-dropouts in liver DWI. Because artifacts can be suppressed locally, no SNR penalties are induced in artifact-free regions. All in all, the proposed approach enables to realize more reliable ADC maps and promises improved assessment of liver DWI in clinical practice.Acknowledgements
We thank the digital health innovation platform (d.hip) for supporting this project.References
[1] Mitchell, D. G., Bruix, J., Sherman, M., & Sirlin, C. B. (2015). LI‐RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI‐RADS Management Working Group and future directions. Hepatology, 61(3), 1056-1065.
[2] Taouli, B., & Koh, D. M. (2010). Diffusion-weighted MR imaging of the liver. Radiology, 254(1), 47-66.
[3] Aliotta, E., Wu, H. H., & Ennis, D. B. (2017). Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion–compensated diffusion‐weighted MRI. Magnetic resonance in medicine, 77(2), 717-729.
[4] Ozaki, M., Inoue, Y., Miyati, T., Hata, H., Mizukami, S., Komi, S., ... & Woodhams, R. (2013). Motion artifact reduction of diffusion‐weighted MRI of the liver: use of velocity‐compensated diffusion gradients combined with tetrahedral gradients. Journal of Magnetic Resonance Imaging, 37(1), 172-178.
[5] Ichikawa, S., Motosugi, U., Tamada, D., Wakayama, T., Sato, K., Funayama, S., & Onishi, H. (2019). Improving the quality of diffusion-weighted imaging of the left hepatic lobe using weighted averaging of signals from multiple excitations. Magnetic Resonance in Medical Sciences, 18(3), 225.
[6] Tamada, D., Motosugi, U., & Onishi, H (2018). Improving the image quality of liver DWI using the convolutional neural network-based selection algorithm. Proceedings of the Internationa Society of Magnetic Resonance in Medicine, #2723.
[7] Zaheer, M., Kottur, S., Ravanbakhsh, S., Poczos, B., Salakhutdinov, R. R., & Smola, A. J. (2017). Deep sets. Advances in neural information processing systems, 30, 3391-3401.
[8] Kingma, D. P., & Ba, J. (2014). Adam: A method for stochastic optimization. arXiv preprint arXiv:1412.6980.
Figures