0522
Skull MRI with MUFFIN: MUlti-Frame Forward-modeled Image Numismatics1Mallinckrodt Institute of Radiology, Washington University in St. Louis, St. Louis, MO, United States, 2Division of Plastic and Reconstructive Surgery, Washington University in St. Louis, St. Louis, MO, United States
Synopsis
Computed tomography (CT) is the reference method for skull imaging, but can cause cancer due to ionizing radiation. Magnetic resonance imaging (MRI) is safer, but the prolonged scan time increases the chance of motion, especially for pediatric patients. Sedation helps reduce motion significantly, but is associated with risks. A sedation-free MRI scheme that is robust to motion is therefore highly desirable. Here, we proposed such a method by making use of a radial acquisition scheme that is inherently robust to motion. The robustness was further boosted by a forward-modeled motion-corrected reconstruction. The results show the promise of the method.
INTRODUCTION
Computed tomography (CT) is the standard of practice for skull imaging. However, the associated ionizing radiation increases the risk of cancer. Magnetic resonance imaging (MRI) offers a safer alternative. A “Black Bone” protocol was previously proposed that obtained dark signal intensities in the bone, enabling the extraction of skull after intensity inversion1,2. However, this method is not widely utilized because of the suboptimal osseous/soft tissue contrast and vulnerability to motion3. Sedation reduces motion, but may lead to medical complications4-7. Therefore, developing a sedation-free MRI scheme that is robust to motion is highly desirable.Radial trajectories are less sensitive to motion than Cartesian ones8. Additionally, multiple short-duration frames can be reconstructed to keep track of motion. Reconstructing multiple frames, inferring transformations between these frames and performing motion correction accordingly were previously employed in many different contexts. Some neuroimaging groups applied motion correction directly in k-space via rotations and phase manipulations9-11. Some other groups performing free-breathing MRI employed the general matrix equation12 to incorporate the deformable motion field into their iterative reconstruction by applying the motion field in the image domain rather than in k-space13,14.
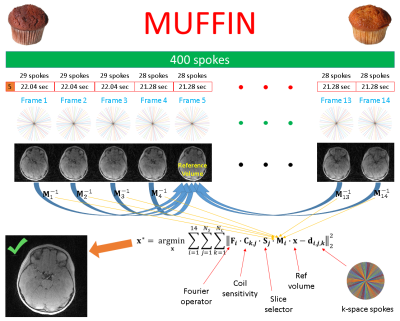
In this study, we used a high-resolution hybrid radial acquisition, estimated rigid body transformations between sliding-window reconstructions and incorporated these transformations into a forward model to collect valuable pieces of information, i.e. to perform “image numismatics”, giving the method its name – MUFFIN: MUlti-Frame Forward-modeled Image Numismatics. The motion-corrected images were post-processed to obtain 3D renderings of the skull. The comparison with the gold standard method (i.e. CT) shows the promise of the method.
METHODS
Upon Institutional Review Board approval, informed consent was obtained from the parent(s) of all participating children. MRI images were acquired using a 3T Prisma, a 3T VIDA, and a 3T Biograph mMR scanner (Siemens Healthcare, Erlangen, Germany). A Fast Low-Angle Shot (FLASH) Golden-Angle 3D stack-of-stars radial VIBE sequence (GA-VIBE)15 was used. The imaging protocol was as follows: TE/TR = 2.47 ms/4.84ms, Bandwidth = 410 Hz/pixel, 224 slices per slab, transverse orientation, Flip angle = 3°, Acquisition matrix = 320 × 320, Voxel size 0.6 x 0.6 x 0.8 mm and number of radial lines = 400 for a scan duration of 5 minutes and 4 seconds. The protocol was designed to maximize the image contrast between bone and all non-osseous tissues by choosing an in-phase TE value and a small flip angle (3°) to increase proton density weighting.For the motion-corrected reconstruction, the first 5 spokes were discarded to reach a steady state. The remaining spokes were divided into 14 non-overlapping volume-frames (the first three were reconstructed using 29 spokes and the rest using 28). 40% Hamming apodization was applied to mitigate the streaking artifacts caused by undersampling. Only the central 75% of the slices were used for image registration to exclude the neck and to save memory. All frames were rigid-body registered onto the first one, and the frame with the rotation vector closest to the mean rotation vector was chosen as the reference. Coil sensitivities were estimated slice by slice using all 395 spokes. MUFFIN tries to solve the following via a conjugate gradient algorithm:
$$\bf{x^{\star}} = arg\min_{\bf{x}}\sum_{i=1}^{14}\sum_{j=1}^{N_s}\sum_{k=1}^{N_c}||\bf{F_i}\cdot\bf{C_{k,j}}\cdot\bf{S_j}\cdot\bf{M_i}\cdot\bf{x}-\bf{d_{i,j,k}}||^{2}_{2}$$
Here, $$$\bf{x}$$$ is the reference volume (i.e. Frame $$$r$$$), $$$\bf{M_i}$$$ is the motion transformation from Frame $$$r$$$ to Frame $$$i$$$, $$$\bf{S_j}$$$ selects Slice $$$j$$$ from the transformed volume, $$$N_s$$$ is the number of slices, $$$\bf{C_{k,j}}$$$ is the coil sensitivity for Coil $$$k$$$ in Slice $$$j$$$, $$$N_c$$$ is the number of coils, $$$\bf{F_i}$$$ is the non-uniform Fast Fourier Transform (NUFFT) operator16 for the set of spokes for Frame $$$i$$$ and $$$\bf{d_{i,j,k}}$$$ is the measured k-space data in the hybrid domain, $$$\bf{d} = \bf{d(k_x,k_y,z)}$$$, obtained by a 1D iFFT along $$$k_z$$$. Figure 1 illustrates MUFFIN.
The CT scans followed standard clinical pediatric methods. A multi-slice Siemens SOMATOM Definition Flash or Force CT scanner (Siemens Medical Systems, Inc., Iselin, NJ) was used with slice thickness ranging from 0.6 mm to 1mm and pixel spacing ranging from 0.31 x 0.31mm to 0.39 x 0.39 mm.
Both MR and CT images were manually processed in 3DSlicer17 for a 3D rendering of the skull.
RESULTS
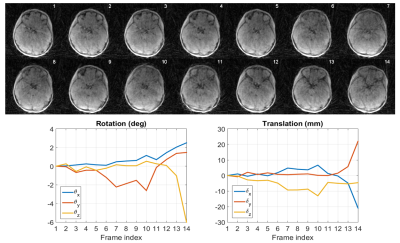
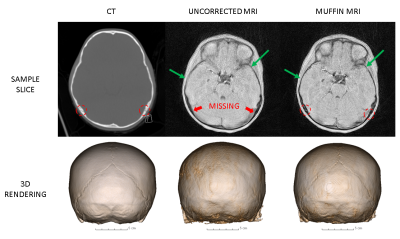
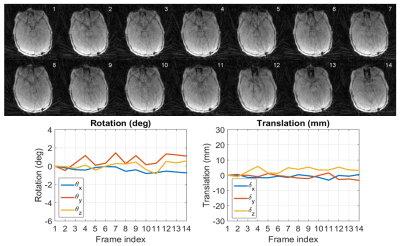
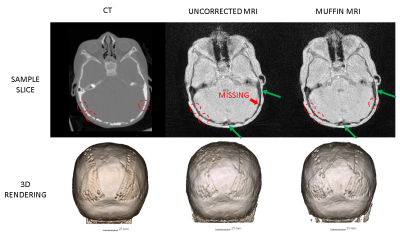
Two patients with considerable motion were chosen for demonstration. Figure 2 depicts, for Patient 1, the low-resolution images used for motion estimation and the estimated rigid-body motion parameters, while Figure 3 exhibits a sample slice and a coronal view of the 3D renderings from the CT scan as well as the uncorrected and corrected MR scans. Figures 4 and 5 show a similar set of results for Patient 2.DISCUSSION
Figures 2-5 make it clear not only that MUFFIN significantly recovers fine structures that can be of critical diagnostic value, but also that the MR-based MUFFIN renderings look extremely similar to the CT-based renderings. If the patient moves significantly within any given frame, however, the artifacts caused by intra-frame inconsistencies may not be recoverable. Shortening the frame duration will help, but will also exacerbate the effects of undersampling.CONCLUSION
MUFFIN may help reduce the use of ionizing radiation and sedation in children. Future work includes improved motion tracking by using higher temporal resolution.Acknowledgements
No acknowledgement found.References
1. Eley KA, Mcintyre AG, Watt-Smith SR, Golding SJ. “Black bone” MRI: a partial flip angle technique for radiation reduction in craniofacial imaging. Br. J. Radiol. 2012;85:272–278
2. Eley KA, Watt-Smith SR, Golding SJ. “Black bone” MRI: a potential alternative to CT when imaging the head and neck: report of eight clinical cases and review of the Oxford experience. Br. J. Radiol. 2012;85:1457–1464.
3. Eley KA, Watt-Smith SR, Golding SJ. Three-Dimensional Reconstruction of the Craniofacial Skeleton With Gradient Echo Magnetic Resonance Imaging (“Black Bone”): What Is Currently Possible? J. Craniofac. Surg. 2017;28:463–467
4. Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics 2000;106:633–644.
5. Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br. J. Anaesth. 2000;84:743–748.
6. Kannikeswaran N, Mahajan PV, Sethuraman U, Groebe A, Chen X. Sedation medication received and adverse events related to sedation for brain MRI in children with and without developmental disabilities. Paediatr. Anaesth. 2009;19:250–256.
7. Uffman JC, Tumin D, Raman V, Thung A, Adler B, Tobias JD. MRI Utilization and the Associated Use of Sedation and Anesthesia in a Pediatric ACO. J. Am. Coll. Radiol. JACR 2017;14:924–930.
8. Glover GH, Pauly JM. Projection Reconstruction Techniques for Reduction of Motion Effects in MRI. Magn. Reson. Med. 1992;28:275–289.
9. Loktyushin A, Nickisch H, Pohmann R, Schölkopf B. Blind retrospective motion correction of MR images. Magn. Reson. Med. 2013;70:1608–1618.
10. Cordero-Grande L, Teixeira RPAG, Hughes EJ, Hutter J, Price AN, Hajnal JV. Sensitivity Encoding for Aligned Multishot Magnetic Resonance Reconstruction. IEEE Trans. Comput. Imaging 2016;2:266–280.
11. Graedel NN, McNab JA, Chiew M, Miller KL. Motion correction for functional MRI with three‐dimensional hybrid radial‐Cartesian EPI. Magn. Reson. Med. 2017;78:527–540.
12. Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn. Reson. Med. 2005;54:1273–1280.
13. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magn. Reson. Med. 2016;75:1484–1498.
14. Cruz G, Atkinson D, Henningsson M, Botnar RM, Prieto C. Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magn. Reson. Med. 2017;77:1894–1908.
15. Peters DC, Korosec FR, Grist TM, et al. Undersampled projection reconstruction applied to MR angiography. Magn. Reson. Med. 2000;43:91–101.
16. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. Ieee Trans. Signal Process. 2003;51:560–574.
17. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012;30:1323–1341.
Figures