0521
Advanced Low-Field MRI of Hip Arthroplasty Implants: First Experience at 0.55 T1Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Siemens Medical Solutions USA Inc., Malvern, PA, United States
Synopsis
Next-generation, advanced low-field MRI holds promise to improve metal artifact reduction MRI of hip arthroplasty implants due to inherently lower susceptibility effects. Using titanium-on-ceramic and metal-on-metal cobalt-chromium total hip arthroplasty implant phantoms, we compared the degree of metal artifacts and signal-to-noise ratios of MR images obtained with modified 0.55T prototype and clinical 1.5T MRI systems. The 0.55T SEMAC MR images with 6-9 encoding steps invariably demonstrated superior, near-complete metal artifact reduction. Our preliminary results suggest clinically viable sequence acquisition times of ≤ 6-min with advanced 0.55T MRI.
Introduction
Based on experience gained from developing high-field magnetic resonance imaging (MRI) systems over the past two decades, next-generation low-field magnetic resonance systems with advanced hardware and software technologies hold great potential to increase healthcare access worldwide, substantially lower cost, flexible utilization, and improved image quality1,2. One major advantage of MRI at low field strengths is reduced susceptibility effects, which are of paramount importance in MRI of metallic orthopedic implants. We aimed to investigate the characteristics of advanced low-field MRI of hip arthroplasty implants at 0.55T, refine MRI protocols intended for clinical use, and compare MRI characteristics with a clinical 1.5T system.Methods
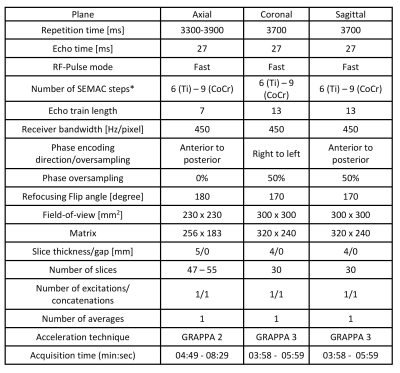
Titanium-on-ceramic (Ti) and metal-on-metal cobalt-chromium (CoCr) total hip arthroplasty implants embedded in standard ASTM gel phantoms were imaged using a commercial MRI system (1.5T MAGNETOM Aera; Siemens Healthcare GmbH, Erlangen, Germany) modified to operate as a prototype system at 0.55T field strength with a gradient strength of 25 mT/m and a slew rate of 40 T/m/sec2. Six-channel body and 18-channel spine array coils tuned to operate at 0.55T were used for signal reception. Turbo spin echo (TSE), view angle tilting (VAT), and combined Slice Encoding for Metal Artifact Correction (SEMAC) with VAT techniques were used. Two full-time board-certified musculoskeletal radiologists qualitatively reviewed various sequence parameters for MRI protocol optimization while taking acquisition times and signal-to-noise ratios (SNR) into consideration. Investigated parameters included SEMAC encoding steps of 6-15, receiver bandwidths of 200-450 Hz/px, refocusing flip angles of 130-180°, and parallel imaging (GRAPPA) acceleration factors of 1-3. Each measurement was repeated twice for SNR measurements using a differential technique3. For comparison with standard 1.5T field strength, the same experimental setup was employed with a clinical 1.5T MRI system (MAGNETOM Sola, Siemens Healthcare) using our clinical hip arthroplasty MRI protocol (SEMAC encoding steps of 11, receiver bandwidth of 504 Hz/px) with 18 channel body and 32-channel spine arrays.Results and Discussion
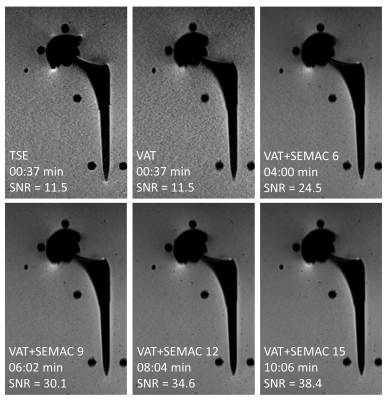
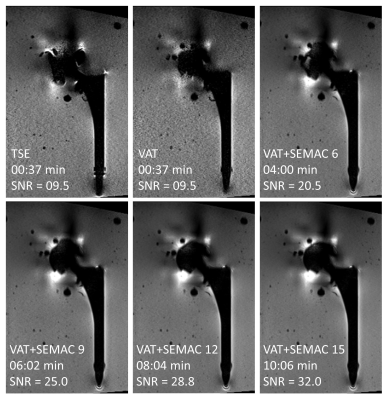
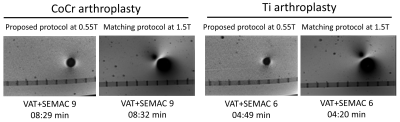
Ti arthroplasty system at 0.55T: SEMAC TSE with six encoding steps produced the maximum metal artifact reduction. A higher number of encoding steps did not further reduce the metal artifacts, despite longer acquisition times. For this type of implant with lower magnetic susceptibility, applying the VAT technique alone without SEMAC may yield satisfactory metal artifact reduction for diagnostic purposes (Figure 1).CoCr arthroplasty system at 0.55T: The cobalt-based alloy's higher magnetic susceptibility required at least nine SEMAC encoding steps to achieve satisfactory metal artifact reduction (Figure 2).
At 0.55T, receiver bandwidths greater than 300 Hz/px had no perceivable effects on further reducing metal artifacts but higher bandwidths were associated with less VAT-induced image blurring, and therefore a bandwidth of 450 Hz/px was overall selected.
Refocusing pulse flip angles of 130-180° had no effect on metal artifacts. Metal artifacts were unaffected by GRAPPA acceleration factors of up to 3. Based on those observations, Table 1 shows the proposed MRI protocol for MRI of hip arthroplasty implants at 0.55T. The number of SEMAC encoding steps may be adjusted if the implant material type is known. Otherwise, a conservative encoding step of 9 can be used for all material types.
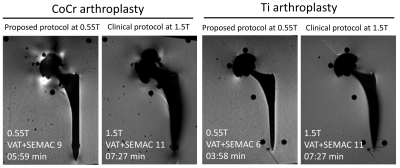
Compared to 1.5T systems, metal-related artifacts at 0.55T systems were invariably smaller (Figures 3 and 4). The advantage of lower field strength was more prominent for the CoCr arthroplasty system, where the metallic artifacts were incompletely reduced at 1.5T due to higher magnetic susceptibility. The lower number of SEMAC steps needed at 0.55T than 1.5T is beneficial to achieve clinically viable acquisition times.
Conclusion
MRI of hip arthroplasty implants using an advanced 0.55T low-field systems is feasible. Applying a systematic protocol optimization algorithm leads to time-efficient sequence acquisition times of 6 min per sequence that appear clinically viable. Hip arthroplasty implant-related susceptibility artifacts are substantially smaller at 0.55T compared to clinical 1.5T field strength.Acknowledgements
The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing research agreement between NYU and Siemens Healthcare.References
1. Runge VM and Heverhagen JT. Advocating the Development of Next-Generation, Advanced-Design Low-Field Magnetic Resonance Systems. Invest Radiol. 2020;55(12):747-753.
2. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019;293(2):384-393.
3. Dietrich O, Raya JG, Reeder SB, et al. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26(2):375-85.
Figures