0518
Pharmacological inactivation of ventral hippocampus disrupts central auditory processing1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China
Synopsis
Audition is vital for communication, learning and memory processes. However, the hippocampus, which can support these functions, is absent from networks of auditory processing. To bridge this gap, we employed auditory fMRI and pharmacological inactivation techniques to directly characterize how hippocampal outputs affect auditory responses to auditory stimuli in primary auditory-associated structures. Using behaviorally-relevant, natural sounds for rodent behaviors, or their temporally-reversed counterparts, we revealed that absence of hippocampal output disrupts auditory responses to vocalizations in auditory midbrain, thalamus and cortex. For the first time, our results demonstrated the critical role of hippocampus in shaping response selectivity to behaviorally-relevant sounds.
Purpose
Communication, learning, and memory require accurate decoding and interpretation of sounds. The hippocampus has well-established roles in these processes1,2, yet its role in auditory processing remains unknown. This is because existing frameworks describing auditory processing often examine fundamental basic features of acoustic stimuli using pure tones and broadband noise3,4. However, these frameworks do not incorporate natural sound processing which requires decoding complex spectrotemporal dynamic properties.Vocalizations are natural sounds that are critical for facilitating behavioral responses and can be innate in rodents5,6. Given its role in memory, emotion, and learning functions1,2,7, the hippocampus, which is also critical for learning and memory1,2, is a strong candidate for inclusion in the auditory processing network. Particularly, the ventral hippocampus (vHP) is associated with emotion/contextual processing, which is likely involved in processing sensory inputs with an emotional context1,2, directly suggesting that auditory processing utilizes sensory-related regions beyond the central auditory pathways. However, whether and how the vHP directly influences vocalization processing remains unknown. Here, we utilized tetrodotoxin (TTX), which blocks the sodium ion channels of vHP neurons to inactivate their activity8, and large-scale fMRI to examine the effects of such manipulation on vocalization processing across the auditory pathway, including the inferior colliculus (IC), medial geniculate body (MGB), and auditory cortex (AC).
Methods
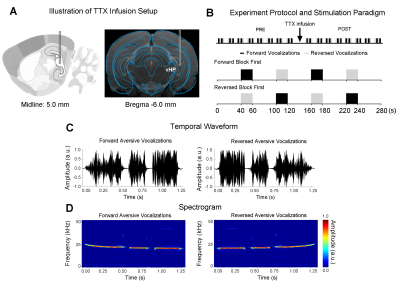
Adult Sprague-Dawley rats (n=7, 12 weeks old, male) were stereotaxically implanted with a cannula (internal diameter: 250μm) in the right/ipsilateral ventral dentate gyrus (vDG) of vHP, to infuse TTX during fMRI experiments (Figure 1A).To investigate the effects of TTX inactivation of vHP neurons on auditory processing, a total of sixteen auditory fMRI sessions were performed in each animal. After eight sessions, 5μL TTX (concentration: 5-10ng/μL)9 was injected into vDG (Figure 1A). The first post scan is acquired one minute after the completion of TTX infusion. During auditory fMRI sessions, auditory stimuli were delivered via a customized tube to the left/contralateral ear. Two stimuli were presented (forward aversive vocalization and temporally reversed aversive vocalizations: 22kHz, 83dB) in a block design paradigm (20s-ON, 40s-OFF, 4 blocks). Auditory fMRI trials starting with forward vocalizations or temporally reversed vocalizations were interleaved (Figure 1B).
All fMRI data was acquired on a 7T Bruker scanner using GE-EPI (FOV=32×32mm, matrix=64×64, α=56°, TE/TR=20/1000ms, twelve 1.0mm slices without gap). Standard fMRI preprocessing was performed before the GLM analysis was applied to identify significant BOLD responses (p<0.001). BOLD signal profiles were extracted from anatomically defined ROI.
Results
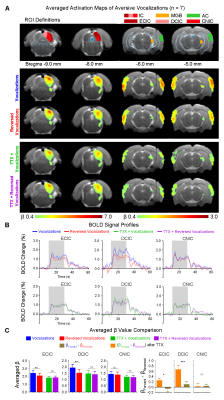
Inactivation of hippocampal outputs disrupts auditory processing of aversive vocalizationsAuditory evoked BOLD responses occurred along central auditory pathways, including ipsilateral IC and MGB, and bilateral AC. Before TTX infusion, responses in IC, MGB and AC were stronger using aversive vocalizations than reversed vocalizations (i.e., with β difference between forward and reversed vocalization responses in IC (p<0.01), MGB(p<0.05) and AC(p<0.05)) (Figure 2). This finding demonstrates the response selectivity to forward vocalizations which corroborates our earlier fMRI study10. Here, the response selectivity to forward aversive vocalizations in IC was most prominent in ECIC (p<0.01) and DCIC (p<0.01), but not CNIC (Figure 3), suggesting that the observed response selectivity to aversive vocalizations likely arise from AC, as ECIC and DCIC receive corticofugal projections from AC11,12.
Interestingly, after TTX infusion into vHP, the response selectivity to forward vocalizations was eliminated throughout the central auditory pathways. Meanwhile, the BOLD responses to forward and reversed vocalizations in IC, MGB and AC were generally diminished. Particularly, the BOLD responses to forward vocalizations were diminished by a greater extent. This finding indicates that the hippocampal outputs selectively modulate auditory responses to forward vocalizations that convey contextual information. Together, our results demonstrate that hippocampal outputs are critical for shaping response selectivity to natural/behaviorally-relevant sounds.
Discussion and Conclusion
In this study, we examine the role of the hippocampal outputs in central auditory processing by monitoring large-scale neural auditory response before and after the infusion of TTX in vHP using brain-wide fMRI. By contrasting the aversive vocalizations with their temporally-reversed counterparts, we revealed the selective influence of hippocampal outputs on auditory processing and its importance in shaping response selectivity to behaviorally-relevant sounds.Here, temporally reversing the vocalizations may alter certain temporal properties so that reversed vocalization no longer carries the critical information embedded within the original spectrotemporal dynamics, which diminishes the behavioral relevance of the sound. Previous studies reported that the hippocampus was recruited to process temporal information of sensory inputs13,14. We postulate that the hippocampus plays a critical role in encoding and recognizing temporal information to subsequently aid in discriminating and interpreting the temporal organization of incoming sensory inputs. Therefore, before TTX infusion, the vHP discriminates and interprets the spectrotemporal dynamics embedded within forward and temporally-reversed vocalizations, and selectively modulate auditory responses, which lead to response selectivity along the auditory pathway. After TTX infusion, the vHP fail to discriminate the spectrotemporal dynamics of incoming auditory inputs, thereby no response selectivity can be established.
Together, we directly reveal the modulatory effects of hippocampal outputs on natural sound processing. The present study expands our current understanding of large-scale central auditory processing beyond the traditional auditory pathways.
Acknowledgements
This study was supported by the Hong Kong Research Grant Council (R7003-19, C7048-16G, HKU17112120, HKU17103819, and HKU17104020), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001), and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001).References
1. Fanselow, M. S. & Dong, H. W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7-19, doi:10.1016/j.neuron.2009.11.031 (2010).
2. Strange, B. A., Witter, M. P., Lein, E. S. & Moser, E. I. Functional organization of the hippocampal longitudinal axis. Nature reviews. Neuroscience 15, 655-669, doi:10.1038/nrn3785 (2014).
3. Kim, G. & Doupe, A. Organized representation of spectrotemporal features in songbird auditory forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 16977-16990, doi:10.1523/JNEUROSCI.2003-11.2011 (2011).
4. Machens, C. K., Wehr, M. S. & Zador, A. M. Linearity of cortical receptive fields measured with natural sounds. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 1089-1100, doi:10.1523/JNEUROSCI.4445-03.2004 (2004).
5. Hammerschmidt, K. et al. Mice do not require auditory input for the normal development of their ultrasonic vocalizations. BMC neuroscience 13, 40, doi:10.1186/1471-2202-13-40 (2012).
6. Mahrt, E. J., Perkel, D. J., Tong, L., Rubel, E. W. & Portfors, C. V. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 5573-5583, doi:10.1523/JNEUROSCI.5054-12.2013 (2013).
7. Bird, C. M. & Burgess, N. The hippocampus and memory: insights from spatial processing. Nature reviews. Neuroscience 9, 182-194, doi:10.1038/nrn2335 (2008).
8. Kaneda, M., Oyama, Y., Ikemoto, Y. & Akaike, N. Blockade of the voltage-dependent sodium current in isolated rat hippocampal neurons by tetrodotoxin and lidocaine. Brain research 484, 348-351, doi:10.1016/0006-8993(89)90379-x (1989).
9. Telensky, P. et al. Functional inactivation of the rat hippocampus disrupts avoidance of a moving object. Proceedings of the National Academy of Sciences of the United States of America 108, 5414-5418, doi:10.1073/pnas.1102525108 (2011).
10. Gao, P. P., Zhang, J. W., Fan, S. J., Sanes, D. H. & Wu, E. X. Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study. NeuroImage 123, 22-32, doi:10.1016/j.neuroimage.2015.08.040 (2015).
11. Xiong, X. R. et al. Auditory cortex controls sound-driven innate defense behaviour through corticofugal projections to inferior colliculus. Nat Commun 6, 7224, doi:10.1038/ncomms8224 (2015).
12. Schofield, B. R. Projections to the inferior colliculus from layer VI cells of auditory cortex. Neuroscience 159, 246-258, doi:10.1016/j.neuroscience.2008.11.013 (2009).
13. Aronov, D., Nevers, R. & Tank, D. W. Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 543, 719-722, doi:10.1038/nature21692 (2017).
14. Eichenbaum, H. Time cells in the hippocampus: a new dimension for mapping memories. Nature reviews. Neuroscience 15, 732-744, doi:10.1038/nrn3827 (2014).
Figures