0517
Investigating Neurophysiological Basis of Resting State fMRI Signal Components through Suppression of Cortical Slow Rhythms1Biomedical Engineering, Emory University/Georgia Institute of Technology, Atlanta, GA, United States, 2Department of Radiology & Imaging Sciences, Emory University, Atlanta, GA, United States
Synopsis
In this study, we tested hypothesis advanced by some groups that brain slow rhythms serve as the neurophysiological basis of resting state fMRI (rsfMRI). Putative suppression of cortical rhythms with an established technique, led to significant reduction in the amplitude of rsfMRI quasi-periodic patterns (QPPs), and enhancement in the rsfMRI measures of intrinsic functional connectivity FC in canonical brain function networks in rats. The results indicate cortical slow rhythms serve as the genesis of only the vigilance dependent components (e.g., QPP) of rsfMRI signals. Further attenuation of these non-specific signals enhances delineation of brain function networks.
Introduction:
The neurophysiological basis underlying resting state fMRI (rsfMRI) signals are not completely understood, impeding accurate interpretation of rsfMRI studies. A number of studies 1,2 point to dynamics of slow rhythms (0.5-2 Hz) as the basis of rsfMRI. Slow rhythms exist in the absence of stimulation, propagate across the cortex 3, and are strongly modulated by vigilance 4 similar to parts of rsfMRI signals 5,6. However, resting state FC can in principle, also be generated through other mechanisms as well, e.g., gamma rhythms 7. Importantly, unlike slow rhythms, the strength of FC generally decreases with reductions in vigilance and arousal levels 8,9. It is possible that slow rhythms provide the basis of only certain (e.g., the vigilant dependent) components of the rsfMRI signal rather than the whole. RsfMRI data exhibit quasi-periodic patterns (QPPs) 5,6 that increase in strength with decreasing vigilance 5,6 and propagate across the brain 10 similar to slow rhythms. QPPs are mostly not specific to, and can confound the accurate estimation of FC in canonical brain function networks. Thus, there is a critical need to examine the effects of manipulation of slow rhythms on rsfMRI. One mechanism for expression and maintenance of cortical slow rhythms in the brain is through a thalamocortical network of coupled oscillators driven by burst firing in thalamus induced by low-threshold T-type calcium (Ca2+) channels (TTCCs) 11,12. Systemic administration of the selective TTCC TTA-P2 13 suppresses cortical slow brain rhythms by up to 60% in anesthetized rats 14. In this study, we examined the effects of TTA-P2 on rsfMRI signal in rats. Our hypotheses were 1) the suppression of slow rhythms engendered by TTA-P2 would reduce the strength of QPPs; 2) which will lead to increased rsfMRI measures of functional connectivity (FC) in canonical brain function networks.Methods:
All experiments were conducted with protocols approved by IACUC. Seven adult Sprague-Dawley rats were administered subcutaneous injections of TTA-P2 (3-6 mg/kg dissolved in Vehicle (4% DMSO saline solution)), immediately after and before 40-90 min fMRI scans obtained under sedation induced by dexmedetomidine (which does not interfere with the action of TTA-P2 15). Three other rats were administered the Vehicle. MRI data were acquired on a 9.4 T Bruker animal MRI system with a custom-built surface coil. RsfMRI scans were obtained with a whole-brain respiration-gated gradient echo EPI (TR/TE/FA = 2000ms/25ms/90°, resolution = 0.5 mm isotropic voxels). RsfMRI preprocessing steps included distortion correction, spatial normalization to standard Paxinos atlas space 16,17, motion parameter regression, and band-pass (0.01-0.20 Hz) filtering. QPP templates were estimated with a well-established technique 18 from the pre-injection (Baseline) fMRI data. The changes in the strength of the expression of QPPs over time for each fMRI scan (Baseline and TTA-P2) for each rat were estimated through the sliding window spatiotemporal correlation (STC) of the corresponding fMRI time-series with that rat’s QPP template. The effects of TTA-P2 on QPPs were assessed with between-session (TTA-P2 vs Baseline) paired t-tests on the mean of positive excursions of the STC curve above zero. FC in brain function networks was assessed through seed-based cross-correlation analysis (sbCCA). A priori seed ROIs for sbCCA were formed encompassing rat barrel cortex (RS1-BF) and auditory cortex (RAud) areas in the right hemisphere. TTA-P2 effects were assessed with between-session t-tests on the z-transformed CC maps; with appropriate multiple comparisons correction (mcc) 19,20.Results & Discussion:
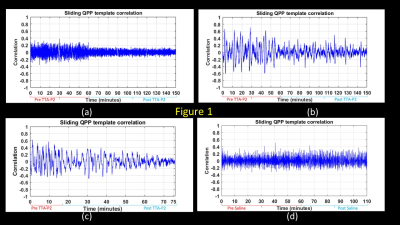
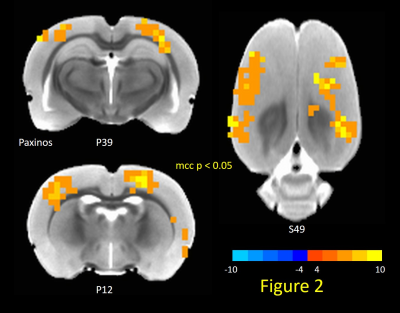
TTA-P2 administration significantly (p < 0.01) reduced the strength (mean of positive STC values) of QPPs compared to Baseline. The amount of suppression of QPPs induced by TTA-P2 varied from 18-58% (mean 48%). Figs.1 (a-c) illustrates this suppression of QPPs in three rats. On the other hand, Vehicle did not change the strength of QPPs significantly. An example of this is provided in Fig.1d. Thus, suppression of cortical slow rhythms (putatively induced by TTA-P2 14) led to expected reduction in the strength of QPPs. This confirms our hypotheses that QPPs are strongly depend on (if not reflect) the expression of cortical slow rhythms. Next, we examined the effect TTA-P2 on FC networks linked to the RS1-BF and RAud in different sbCCAs. TTA-P2 significantly increased the rsfMRI FC between RS1-BF (Fig.2) and some areas in somatosensory, motor, auditory, visual, and parietal cortices, bilaterally consistent with increased in corresponding canonical brain function networks 21-24. RAud exhibited (Fig.3) significantly increased FC to contralateral auditory, visual and somatosensory areas which enhanced the delineation of related brain circuits 23,25,26. Vehicle administration did not evoke appreciable changes in FC. Thus, putative suppression of cortical slow rhythms induced by TTA-P2 increased FC in canonical brain function networks as expected due to the reduction of non-specific QPP signals.Conclusion:
The results indicate that the vigilance dependent components of the rsfMRI signal (e.g. QPPs) reflect the dynamics of cortical slow rhythms. Suppression of slow rhythms reduces the strength of vigilance dependent rsfMRI signals and enhances FC derived from rsfMRI in canonical brain function networks. These results have profound implications to our understanding of neurophysiological basis of rsfMRI signals. Future work would include simultaneous EEG recordings to directly examine cortical slow rhythms, and intra-thalamic administration of TTA-P2 to specifically target only TTCCs part of thalamocortical slow wave generating unit.Acknowledgements
This work was supported by Radiology Seed Grants from Department of Radiology & Imaging Sciences, Emory UniversityReferences
1. Chan RW, Leong ATL, Ho LC, et al. Low-frequency hippocampal-cortical activity drives brain-wide resting-state functional MRI connectivity. Proc Natl Acad Sci U S A 2017.
2. Matsui T, Murakami T, Ohki K. Transient neuronal coactivations embedded in globally propagating waves underlie resting-state functional connectivity. Proc Natl Acad Sci U S A 2016;113:6556-61.
3. Sheroziya M, Timofeev I. Global intracellular slow-wave dynamics of the thalamocortical system. J Neurosci 2014;34:8875-93.
4. Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience 2000;101:243-76.
5. Billings JCW, Keilholz SD. The Not-So-Global BOLD Signal. Brain Connect 2018.
6. Thompson GJ, Magnuson ME, Merritt MD, et al. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp 2013;34:3280-98.
7. Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A 2010;107:10238-43.
8. Hutchison RM, Hutchison M, Manning KY, Menon RS, Everling S. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain's functional architecture. Hum Brain Mapp 2014.
9. Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A 2009;106:4489-94.
10. Majeed W, Magnuson M, Keilholz SD. Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat. J Magn Reson Imaging 2009;30:384-93.
11. Crunelli V, David F, Lorincz ML, Hughes SW. The thalamocortical network as a single slow wave-generating unit. Curr Opin Neurobiol 2015;31:72-80.
12. Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci 2010;13:9-17.
13. Dreyfus FM, Tscherter A, Errington AC, et al. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci 2010;30:99-109.
14. David F, Schmiedt JT, Taylor HL, et al. Essential thalamic contribution to slow waves of natural sleep. J Neurosci 2013;33:19599-610.
15. Horvath G, Morvay Z, Kovacs M, Szilagyi A, Szikszay M. Drugs acting on calcium channels modulate the diuretic and micturition effects of dexmedetomidine in rats. Life Sci 1996;59:1247-57.
16. Paxinos G, Watson C. Paxino's and Watson's The rat brain in stereotaxic coordinates. Seventh edition. ed. Amsterdam ; Boston: Elsevier/AP, Academic Press is an imprint of Elsevier; 2014.
17. Valdes-Hernandez PA, Sumiyoshi A, Nonaka H, et al. An in vivo MRI Template Set for Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front Neuroinform 2011;5:26.
18. Majeed W, Magnuson M, Hasenkamp W, et al. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. Neuroimage 2011;54:1140-50.
19. Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 2017;7:152-71.
20. Gopinath K, Krishnamurthy V, Sathian K. Accounting for Non-Gaussian Sources of Spatial Correlation in Parametric Functional Magnetic Resonance Imaging Paradigms I: Revisiting Cluster-Based Inferences. Brain Connect 2018;8:1-9.
21. Rao RP, Mielke F, Bobrov E, Brecht M. Vocalization-whisking coordination and multisensory integration of social signals in rat auditory cortex. eLife 2014;3.
22. Frostig RD, Xiong Y, Chen-Bee CH, Kvasnak E, Stehberg J. Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J Neurosci 2008;28:13274-84.
23. Reid JM, Jacklin DL, Winters BD. Crossmodal object recognition in rats with and without multimodal object pre-exposure: no effect of hippocampal lesions. Neurobiol Learn Mem 2012;98:311-9.
24. Zakiewicz IM, Bjaalie JG, Leergaard TB. Brain-wide map of efferent projections from rat barrel cortex. Front Neuroinform 2014;8:5.
25. Schormans AL, Scott KE, Vo AM, et al. Audiovisual Temporal Processing and Synchrony Perception in the Rat. Frontiers in behavioral neuroscience 2016;10:246.
26. Thomas ME, Lane CP, Chaudron YMJ, Cisneros-Franco JM, de Villers-Sidani E. Modifying the Adult Rat Tonotopic Map with Sound Exposure Produces Frequency Discrimination Deficits That Are Recovered with Training. J Neurosci 2020;40:2259-68.
Figures