0509
MB-SWIFT fMRI studies in head-fixed behaving rats1A.I.V. Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland, 2Grenoble Institut des Neurosciences, Grenoble, France, 3Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Currently there are no tools to study simultaneously whole-brain processing and behavior in rats. Here we introduce a novel approach for fMRI studies in head-fixed and minimally restrained rats that can express behavior. This was achieved with MB-SWIFT sequence that is both quiet and insensitive to movement. First, the whole brain, including hindbrain, was functionally parcellated with high fidelity. Second, fMRI maps showing activation in relevant networks during spontaneous single behavioral events were successfully generated. Our approach links global network activity to behavior and has potential to enable novel experimental designs in neuroscience studies.
Introduction
Understanding normal and abnormal brain and mind, and their link to behavior is one of the key challenges of modern neuroscience. Rodent studies are well-suited for this purpose, as rodents exhibit many basic aspects of mammal behavior and cognitive processing1-2. This relationship has been typically studied with electrophysiological recordings in head-restrained or freely moving rodents1. Positron emission tomography (PET)3-4, optical imaging1, and focused ultrasound (fUS)5 have also been exploited. While electrophysiological and optical measurements provide high temporal resolution, they suffer from limited spatial coverage. PET provides whole-brain coverage, but suffers from low temporal resolution. fUS allows high temporal and spatial resolution, but whole-brain coverage has been demonstrated only in anesthetized animals6. Thus, behavioral studies currently lack tools to provide insights into how the brain modulates at network-level during specific tasks.Awake rodent functional MRI (fMRI) has gained increasing interest in neuroscience as it provides good spatial and temporal resolution with whole-brain coverage7-9. However, the subject is typically restrained to minimize motion-induced artefacts, making behavioral studies not achievable. To overcome many limitations of the traditional EPI fMRI, we introduced Multi-Band SWeep Imaging with Fourier Transformation (MB-SWIFT)10 in the context of awake rat fMRI11. MB-SWIFT is ideal for awake fMRI studies as it provides similar or even better functional contrast as compared to spin echo EPI12, good correlation with neuronal activity11, and is also quiet and insensitive to movement- and susceptibility-induced artefacts11. As a continuation of our previous studies, the aim of this work was to demonstrate that MB-SWIFT technique enables studies in head-fixed rats expressing spontaneous behavior.
Methods
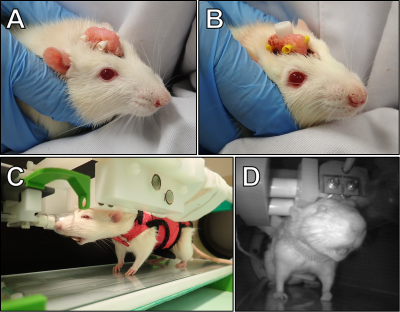
Animal procedures were approved by the Animal Experiment Board in Finland. Adult (448 ± 56 g) male Sprague-Dawley rats (n = 10) were used. An implant for head-fixation (Figure 1A) was attached as described earlier11. For one rat, electroencephalography (EEG) electrodes were implanted to test the feasibility of behavioral EEG/fMRI (Figure 1B). The rats were habituated to fMRI for 9 days, with periodic corticosterone level blood sampling. Rats wore a soft walking harness and ear plugs (Figure 1C). The harness allowed standing, sitting, and body and limb movements, yet prevented excessive forces to the head implant. During fMRI, the rat behavior was recorded with an MRI-compatible video camera (Figure 1D), and breathing was monitored with a sensor pad placed inside the harness.MRI was performed as previously described11. An Agilent 9.4 T magnet with a 21-cm bore and a custom-made transmit-receive loop coil (22 mm inner diameter) were used. Anatomical images were obtained with a fast spin echo sequence. fMRI data were acquired with a 3D MB-SWIFT (2000 spokes, TR 0.97 ms, temporal resolution ~2 s, 192/384 kHz excitation/acquisition bandwidths, 6° flip angle, and 643 matrix size with 625 µm isotropic resolution). Each rat underwent fMRI 1-3 times (15-25 min, 450-750 fMRI volumes each). Positioning to the holder and MRI adjustments were performed while the rats were under sevoflurane (2-3%) anesthesia. After turning sevoflurane off, fMRI was started when the rat either moved or its breathing rate increased >100 BPM. Data were processed and analyzed as described earlier11.
Results
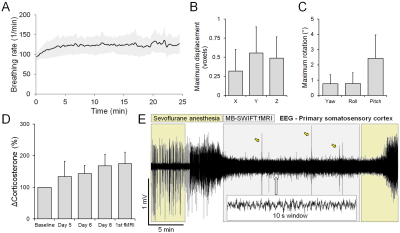
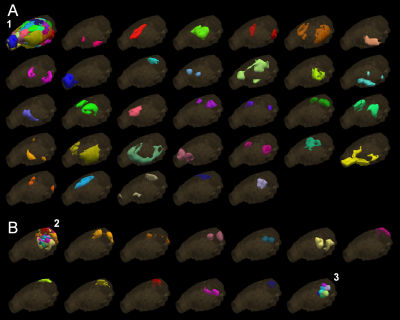
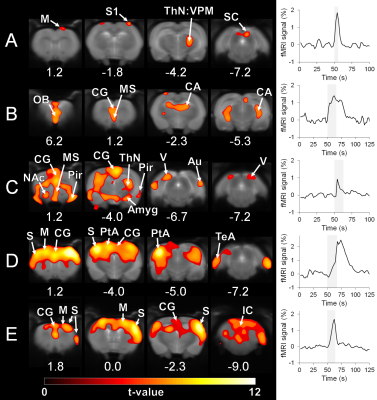
Rat breathing rates remained stable during the awake fMRI (Figure 2A; average 121 ± 23 BPM). Head movement occurred at the rate of 0.32 ± 0.19 events / min, during which the translation remained minimal (Figure 2B and 2C). A moderate increase in the corticosterone levels (Figure 2D, 74.7 ± 36.9%; p<0.05, paired Student´s t-test) and small decrease in body weight (2.6 ± 1.4 %; p<0.05, paired Student´s t-test) were observed during the training period.A representative EEG time series is shown in Figure 2E, with a clear difference between anesthetized and awake conditions. In the preliminary fMRI data analyses, an excellent functional parcellation was achieved, including the hindbrain (Figure 3). Additionally, Figure 4 shows activation patterns in relevant brain areas and networks obtained during single spontaneous behavioral events, such as in somatosensory cortex and ventral posteromedial thalamic nucleus during whisking, in olfactory bulb during sniffing, in visual and auditory cortices during alertness, and in somatosensory and motor cortex during limb movement.
Discussion
For the first time, high-quality EEG/fMRI data were obtained from head-fixed spontaneously behaving rats. The excellent functional parcellation appears similar or better to one obtained earlier in restrained animals11. Importantly, the method was sensitive to spontaneous single behavioral events as demonstrated by activations of relevant brain regions and networks obtained with general linear model block analysis. More advanced analysis is required to extract information about relationship between more complex behavior and global brain activity.A slight decrease in body weight and increased corticosterone levels were observed, potentially indicating increased stress levels. However, the 2-3% change in weight can be considered negligible, and the 0.7-fold increase in corticosterone levels is mild given that other awake rat fMRI protocols have reported 3-6-fold changes13-14 and that stress models using body restrainer often report 10-fold changes15. Nevertheless, the habituation protocol may require further optimization.
Conclusion
We conclude that the novel approach provides a robust and sensitive tool for the brain network-level behavioural studies and can be combined with electrophysiological measurements.Acknowledgements
This work was supported by the National Institutes of Health (U01-NS103569-01 and P41-EB027061), and Jane & Aatos Erkko Foundation.References
1. Ziv Y, Ghosh KK. Miniature microscopes for large-scale imaging of neuronal activity in freely behaving rodents. Curr Opin Neurobiol. 2015 Jun;32:141-7.
2. Hanks TD, Summerfield C. Perceptual Decision Making in Rodents, Monkeys, and Humans. Neuron. 2017 Jan 4;93(1):15-31.
3. Kerr JN, Nimmerjahn A. Functional imaging in freely moving animals. Curr Opin Neurobiol. 2012 Feb;22(1):45-53.
4. Kyme AZ, Angelis GI, Eisenhuth J, et al. Open-field PET: Simultaneous brain functional imaging and behavioural response measurements in freely moving small animals. Neuroimage. 2019 Mar;188:92-101.
5. Urban A, Dussaux C, Martel G, et al. Real-time imaging of brain activity in freely moving rats using functional ultrasound. Nat Methods. 2015 Sep;12(9):873-8.
6. Rabut C, Correia M, Finel V, et al. 4D functional ultrasound imaging of whole-brain activity in rodents. Nat Methods. 2019 Oct;16(10):994-997.
7. Becerra L, Pendse G, Chang PC, et al. Robust reproducible resting state networks in the awake rodent brain. PLoS One. 2011;6(10):e25701.
8. Liang Z, King J, Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci. 2011 Mar 9;31(10):3776-83.
9. Stenroos P, Paasonen J, Salo RA, et al. Awake Rat Brain Functional Magnetic Resonance Imaging Using Standard Radio Frequency Coils and a 3D Printed Restraint Kit. Front Neurosci. 2018 Aug 20;12:548.
10. Idiyatullin D, Corum CA, Garwood M. Multi-Band-SWIFT. J Magn Reson. 2015 Feb;251:19-25.
11. Paasonen J, Laakso H, Pirttimäki T, et al. Multi-band SWIFT enables quiet and artefact-free EEG-fMRI and awake fMRI studies in rat. Neuroimage. 2020 Feb 1;206:116338.
12. Lehto LJ, Idiyatullin D, Zhang J, et al. MB-SWIFT functional MRI during deep brain stimulation in rats. Neuroimage. 2017 Oct 1;159:443-448.
13. King JA, Garelick TS, Brevard ME, et al. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005 Oct 30;148(2):154-60.
14. Chang PC, Procissi D, Bao Q, et al. Novel method for functional brain imaging in awake minimally restrained rats. J Neurophysiol. 2016 Jul 1;116(1):61-80.
15. García-Iglesias BB, Mendoza-Garrido ME, Gutiérrez-Ospina G, et al. Sensitization of restraint-induced corticosterone secretion after chronic restraint in rats: involvement of 5-HT₇ receptors. Neuropharmacology. 2013 Aug;71:216-27.
Figures