0508
The key to extremely accelerated model-based quantitative first-pass perfusion cardiac MRI1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom
Synopsis
First-pass perfusion cardiac MR (FP-CMR) is one of the methods of choice for evaluating myocardial ischemia. Moreover, quantitative FP-CMR methods that provide pixel-wise quantitative myocardial perfusion maps are increasingly being applied as an alternative to visual inspection. Recently, a DIRect QuanTitative (DIREQT) FP-CMR model-based reconstruction has been proposed to directly estimate myocardial perfusion maps and highly accelerate FP-CMR scans. Here, DIREQT is combined with the idea of view-sharing and KEYhole imaging (DIREQT-KEY) to improve DIREQT reconstructions, particularly from extremely accelerated FP-CMR acquisitions. DIREQT-KEY directly generates high-quality quantitative myocardial perfusion maps from less than 4 radial spokes per time frame.
Introduction
First-pass perfusion cardiac MR (FP-CMR) is an emerging non-invasive tool for evaluating coronary heart disease (CHD). Recently, FP-CMR methods that provide pixel-wise quantification of myocardial perfusion have gained increased interest as an alternative to visual inspection.1-3 Typically, these methods indirectly generate quantitative myocardial perfusion maps by first reconstructing individual dynamic contrast-enhanced images, which are then converted to contrast agent concentration and, finally, tracer-kinetic (TK) modeling is used to generate quantitative maps. However, FP-CMR time frames must be acquired in real-time to capture the rapid passage of a contrast agent bolus through the heart, and hence, the spatial resolution and coverage of the heart is compromised.4,5 Undersampled reconstruction methods have been proposed to moderately accelerate FP-CMR acquisitions as a means to improve spatial resolution.6-9 Recently, a DIRect QuanTitative (DIREQT) FP-CMR reconstruction method has been proposed to directly estimate quantitative TK maps from highly undersampled acquisitions, by exploiting the redundancy of spatial information between time frames.10 However, some TK models only use the key frames of contrast arrival for quantitative analysis. Nonetheless, high spatial frequencies from the k-space periphery of the non-key frames (or additionally acquired frames) may contain useful information that could be shared to improve DIREQT reconstructions from highly accelerated FP-CMR data. In this work, DIREQT is combined with the idea of view-sharing and KEYhole imaging11 (DIREQT-KEY) to improve the quality of the TK maps, particularly from extremely accelerated FP-CMR acquisitions. The proposed method was tested in four patients with suspected CHD.Methods
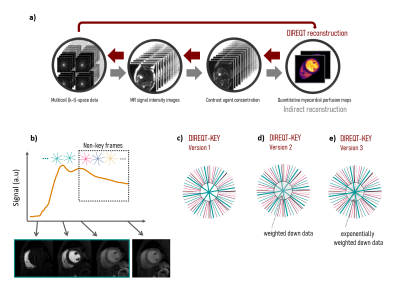
Acquisition Rest FP-CMR fully-sampled acquisitions were performed in four patients with suspected CHD (ethically approved; informed written consent obtained) using a dual-bolus technique with 0.0075+0.075mmol/kg Gadobutrol (Gadovist; Bayer, Germany). Patients 1&2 were scanned on a 3T Philips Achieva scanner and patients 3&4 were scanned on a 1.5T Philips Ingenia scanner (Philips Healthcare, Netherlands). A saturation-recovery turbo field echo (TFE) sequence was used to acquire one short-axis slice in free-breathing using the following parameters: FOV=320x320mm2, resolution=2.8x2.8mm2, slice thickness=10mm, TS/TR/TE= 120/1.96/0.93ms, flip angle=15deg, acquisition window=224-228ms, total acquisition time=1min20s, dynamic frames=90-125, and contrast agent relaxivity=5.0 L/mmol·s. The AIF was found using a region of interest in the left ventricle. The fully-sampled dynamic images were used to estimate the frame-to-frame translational motion. Then, translational motion correction was performed directly in k-space to generate motion-corrected datasets.Reconstruction Radial (k-t)-space sampling was used to generate 40x (3 spokes), 60x (2 spokes) and 120x (1 spoke) undersampled datasets, which were reconstructed using DIREQT and DIREQT-KEY (Fig.1). The DIREQT method directly estimates TK parameter maps from the measured multicoil (k-t)-space FP-CMR data. This is achieved by solving the following optimization problem: $$$\widehat{\textbf{M}}=\underset{\textbf{M}}{\arg\min}\parallel\textbf{d}-f(\textbf{M})\parallel_2^2$$$, where $$$\textbf{M}$$$ are the TK parameters maps (e.g. KTrans and vp of the Patlak model), $$$\textbf{d}$$$ is the undersampled (k-t)-space data and $$$f$$$ is the forward model (indicated by the small red arrows in Fig. 1a). A limited memory BFGS quasi-Newton method was used to solve this nonlinear inverse problem. Three different versions of DIREQT-KEY were tested (Fig. 1b): 1) a central “keyhole” keeps the acquired (k-t)-space data and the peripheral data from the non-key frames are shared; 2) same as 1, but the central region also shares the weighted down (k-t)-space data from the non-key frames; 3) same as 1, but exponentially weighted down data from adjacent times frames is used in each “keyhole". In the latter, most weights will be closer to zero, except for the 4 closest time frames.
Results and Discussion
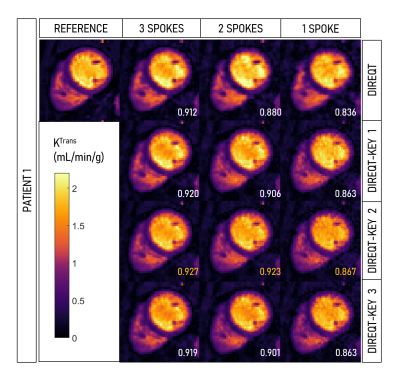
Figure 2 shows the DIREQT and DIREQT-KEY reconstructions obtained for patient 1. The structural similarity index (SSIM) indicates that all three versions of DIREQT-KEY generate TK maps with superior image quality compared to the DIREQT method. The highest SSIM was achieved with DIREQT-KEY version 2, which indicates a better agreement with the reference. Hence, the remaining analysis was done using DIREQT-KEY version 2. Figure 3 shows the quantitative maps obtained for patients 2-4 using the DIREQT and DIREQT-KEY methods. Patient 2 had myocardial infarction and patient 4 hypertrophic cardiomyopathy. Patients 1 and 3 had no abnormalities on CMR. The perfusion defect in Patient 2 was identified in both DIREQT and DIREQT-KEY maps and corresponds to an area of myocardial scarring. DIREQT-KEY improvements are more noticeable for Patient 1&4 since these had more extra frames. However, additional time frames could be acquired to improve DIREQT-KEY maps (Fig. 4-5). DIREQT and DIREQT-KEY provide high-quality maps even at extremely high acceleration rates (Fig. 3-5). However, DIREQT-KEY provides the highest (SSIM) quality TK maps (Fig. 5). Spatial regularization could be used to reduce noise amplification at high accelerations.Conclusion
The proposed DIREQT-KEY, combines DIREQT, a model-based reconstruction approach, with view-sharing and keyhole imaging. Similar to DIREQT, DIREQT-KEY allows extremely high FP-CMR scan acceleration factors (< 4 radial spokes) and quantitative imaging. However, DIREQT-KEY provides higher quality TK maps. Moreover, DIREQT-KEY works with existing (k,t)-sampling, dual-bolus and dual-sequence acquisitions strategies. These aspects enhance the clinical applicability of the method. In future studies, the DIREQT-KEY method will be evaluated in a large cohort of patients with suspected CHD using prospectively undersampled acquisitions. These studies will also aim to achieve much higher spatial resolution and coverage, and hence, greater diagnostic accuracy.Acknowledgements
This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z].References
1. Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19(1):43.
2. Hsu LY, Jacobs M, Benovoy M, et al. Diagnostic performance of fully automated pixel-wise quantitative myocardial perfusion imaging by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2018;11(5):697-707.
3. Villa ADM, Corsinovi L, Ntalas I, et al. Importance of operator training and rest perfusion on the diagnostic accuracy of stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2018;20(1):74.
4. Kellman P, Arai AE. Imaging sequences for first pass perfusion - a review. J Cardiovasc Magn Reson. 2007;9(3):525-37.
5. Fair MJ, Gatehouse PD, DiBella EV, Firmin DN. A review of 3D first-pass, whole-heart, myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9(3):525-37.
6. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64(3):767-76.
7. Vitanis V, Manka R, Giese D, et al. High resolution three-dimensional cardiac perfusion imaging using compartment-based k-t principal component analysis. Magn Reson Med. 2011;65(2):575-87.
8. Lingala SG, Hu Y, DiBella E, Jacob M. Accelerated dynamic MRI exploiting sparsity and low-rank structure: k-t SLR. IEEE Trans Med Imaging. 2011;30(5):1042-54.
9. Chen X, Salerno M, Yang Y, Epstein FH. Motion-compensated compressed sensing for dynamic contrast-enhanced MRI using regional spatiotemporal sparsity and region tracking: block low-rank sparsity with motion-guidance (BLOSM). Magn Reson Med. 2014;72(4):1028-38.
10. Correia T, Schneider T, Chiribiri A. Model-Based Reconstruction for Highly Accelerated First-Pass Perfusion Cardiac MRI. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019. Lecture Notes in Computer Science, Springer. 2019;11765.
11. van Vaals J, Brummer M, Dixon W, et al. ‘Keyhole’ method for accelerating imaging of contrast agent uptake. J Magn Reson Imaging 1993;3: 671-5.
Figures