0507
Flow and Motion Insensitive Steady State (FAMISS): Advancing ungated steady-state cardiac perfusion1Radiology and Imaging Sciences, University Of Utah, Salt Lake City, UT, United States, 2Cardiovascular Medicine, University Of Utah, Salt Lake City, UT, United States
Synopsis
Quantitative cardiac perfusion requires reliable dynamic measurement of the contrast agent concentration in both myocardium and blood. Ungated methods offer immunity to poor ECG signals, acquire multiple cardiac phases and have been shown to achieve comparable contrast-to-noise as saturation recovery based methods. However, blood flow and cardiac motion can interrupt the steady-state and result in the calculation of inaccurate contrast agent concentrations. These errors in perfusion quantification can be avoided using a proposed Flow and Motion Insensitive Steady State (FAMISS) technique.
Introduction
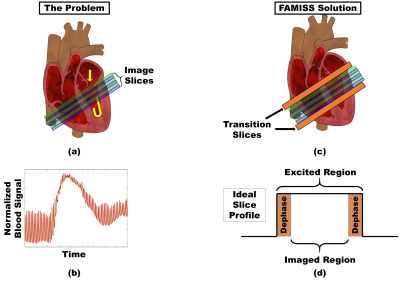
To quantify cardiac perfusion using an ungated steady-state spoiled gradient echo sequences requires both myocardium and blood to have a consistent excitation history (1). However, due to blood flow and cardiac motion, tissue moving into an excited slice may not have the same excitation history as indigenous tissue (Figures 1a and 1b). This problem results in the calculation of inaccurate contrast agent concentrations, most notable in the edge slices and blood pool regions, which culminate in perfusion quantification errors. Consequently, edge slices are frequently acquired but discarded and an independent method is often used to quantify blood contrast agent concentration (2). The proposed solution uses a Flow and Motion Insensitive Steady State (FAMISS) technique to simultaneously excite transition slices (Figure 1c) with no added scan time. Very little work has been done in this area. Sharif et al. proposed a technique that inserts a periodic dummy pulse at the expense of increased scan time (3) while the method by Ragan et al. (4) can suffer from incomplete transition slice dephasing and is not well suited for simultaneous multislice excitation. The relevance of FAMISS to ungated cardiac perfusion is improved precision and coverage with no need for an independent method to quantify blood contrast agent concentration.Method
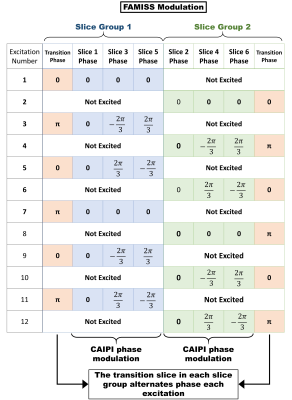
All studies were performed on either a Prisma or Vida 3T MRI scanner (Siemens Healthcare, Erlangen, DE) with all human studies approved by the University of Utah institutional review board and animal studies approved by the Institutional Animal Care and Use Committee. The FAMISS technique is based on a 2D radial FLASH imaging sequence with a TE/TR of 0.75ms/2.1ms, 1.8mm x 1.8mm x 7mm acquisition resolution, 6 slices acquired in two groups (making the effective TR 4.2ms), radial CAIPIRINHA (5) with a simultaneous multiband factor of 3, 8° flip angle and a Golden Angle radial distribution of rays. Images were reconstructed using a motion-compensated patch-based locally low-rank reconstruction technique (2). Contrast animal studies used 0.06 mmol/kg of Gadoteridol (ProHance, Bracco Diagnostics) while human studies used 0.075 mmol/kg of Gadobutrol (Gadavist, Bayer). The FAMISS technique adds a simultaneous excitation of transition slices to the excitation of the image slices (Figures 1c and 1d). The excited signal from each transition slice is removed from the imaging signal using gradient dephasing (Figure 2) and RF phase cycling (Figure 3). Because the transition slices are excited and dephased simultaneously with the image slice excitation, no added echo or scan time is required. There is an increase in power deposition due to the added RF excitation and the total RF duration was 768us.Results
While preliminary data has been acquired in four animals (acquired on a Prisma MRI scanner) and two human cardiac studies (acquired on a Vida MRI scanner), only one representative animal study is shown to illustrate the effect of FAMISS for this abstract. Image comparison between the FLASH and FAMISS sequences, at peak blood pool contrast agent concentration, show no remarkable differences (Figure 4). Blood pool signals for the FLASH sequence show significant pulsatility (Figure 1b). Differences between systole and diastole blood pool signal are shown across the injection of contrast agent except during peak contrast uptake (Figure 4a). However, the FAMISS sequence shows similar blood pool signals along all parts of the contrast curve (Figure 4b) with little to no pulsatility.Discussion
During peak contrast agent concentration in the blood pool, the very short T1 relaxation of blood reduces the effect of excitation history on blood signal. It is therefore expected that systolic and diastolic images would have similar blood to myocardium contrast despite different blood excitation histories. In addition, since the FAMISS transition slices work to preserve excitation history, they have little effect during peak contrast agent concentration in the blood pool. Consequently, the main purpose in showing the images in Figure 4 is to demonstrate that the excited FAMISS transition slices do not contribute any artifacts to the image slices (compare FLASH and FAMISS images in Figure 4). For the FLASH sequence during diastole, the inflow of blood into the left ventricle results in flow-related blood pool enhancement (red line in Figure 4a). The amount of enhancement (difference between red and blue lines in Figure 4a) decreases as the slices become more apical since the incoming blood must first pass through the basal slices and is driven back into steady-state before reaching the apical slices. During systole, there is less of an effect since there is little blood entering the left ventricle during this cardiac phase. The FAMISS plots (Figure 4b) show little to no flow-related enhancement of the blood pool signal in either cardiac phase as expected.Conclusion
This work illustrates that the FAMISS technique will allow improved quantification of blood contrast agent concentration and will eliminate the need to discard edge slices due to cardiac motion in ungated steady-state sequences.Acknowledgements
Supported by the National Institutes of Health R01HL138082 and equipment obtained through S10OD018482-01. Animal studies were supported in part by R01HL142913.References
1. DiBella EV, Chen L, Schabel MC, Adluru G, McGann CJ. Myocardial perfusion acquisition without magnetization preparation or gating. Magn Reson Med. 2012;67:609-613.
2. Tian, Y, Mendes, J, Wilson, B, et al. Whole‐heart, ungated, free‐breathing, cardiac‐phase‐resolved myocardial perfusion MRI by using Continuous Radial Interleaved simultaneous Multi‐slice acquisitions at sPoiled steady‐state (CRIMP). Magn Reson Med. 2020; 84: 3071– 3087.
3. Sharif et al. End-systolic Myocardial Perfusion MRI Using a Hybrid 2D/3D Steady-State Acquisition Scheme: Towards Reliable Detection of Subendocardial Ischemia in Coronary Microvascular Dysfunction. Proc. Intl. Soc. Mag. Reson. Med. 24 (2016), p. 467.
4. Ragan DK, Bankson JA. Suppression of vascular enhancement artifacts through the use of a multiband, selectively spoiled radiofrequency excitation pulse. J Magn Reson Imaging. 2011 May;33(5):1256-61.
5. Yutzy SR, Seiberlich N, Duerk JL, Griswold MA. Improvements in multislice parallel imaging using radial CAIPIRINHA. Magn Reson Med. 2011;65:1630-1637.
Figures