0506
Real-time Cardiac MRI during Exercise with Radial Sampling and Compressed Sensing: Evaluation in a Numerical Phantom and In-Vivo1University of Wisconsin-Madison, Madison, WI, United States, 2Mayo Clinic, Rochester, MN, United States
Synopsis
We used radial sampling, parallel imaging, and compressed sensing for real-time cardiac MRI during exercise, comparing the performance of two temporal resolutions for this approach in a numerical phantom and in a human volunteer. We found the approach feasible with sufficient spatial and temporal resolution to capture myocardial motion. While a longer temporal resolution with 30 radial spokes provides better image quality during rest, shortening the temporal resolution by acquiring just 20 spokes can improve results during exercise by better capturing rapid motion such as late diastolic filling.
INTRODUCTION
Cardiac MRI (CMR) is a powerful tool for assessing myocardial anatomy and function including volumes and myocardial motion1. CMR during exercise in the bore to probe physiological responses to exercise stress in real time2 is of clinical interest yet challenging due to subject motion, need for free-breathing acquisitions, and compromised ECG quality. ‘Realtime’ imaging instead of cine is promising to address these challenges but requires particularly high temporal resolution due to high heart rates, producing a trade-off between temporal resolution, spatial resolution, and undersampling artifact. Prior approaches have used parallel imaging2, non-cartesian sampling3, and constrained reconstructions4 to push the limits of spatiotemporal resolution. However, the tradeoffs between temporal resolution and undersampling artifact during exercise remain under-investigated. In this pilot study, we employed radial undersampling, parallel imaging, and compressed sensing for real-time CMR during exercise and analyzed the performance of this approach in a numerical phantom and in a human volunteer.METHODS
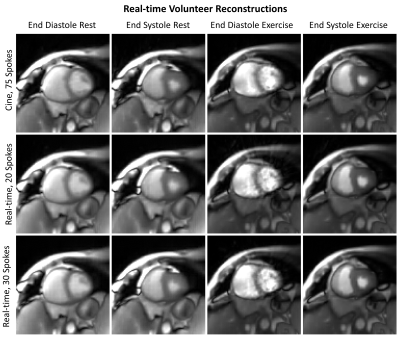
A 2D MRI sequence with balanced steady-state free procession (bSSFP) contrast and tiny golden angle view ordering5 was used in a MRXCAT6 numerical phantom capable to simulate cardiac and respiratory motion and in a 70-year-old volunteer from a study probing the exercise response in pulmonary hypertension (PH) patients. The results of that PH study are beyond the scope of this abstract, which focusses on MRI technical development. The MRXCAT simulation used heart rates of 60 and 120 bpm and respiratory rates of 12 and 24 bpm to simulate rest and exercise, respectively, and 8 simulated coil channels. The volunteer was imaged on a 3T scanner (Discovery MR750, GE Healthcare, Waukesha, WI) with an 8-channel cardiac coil at rest and during exercise with a pneumatic MRI-compatible exercise stepper (Cardio Step Module; Ergospect, Innsbruck, Austria) with stepping resistance automatically adjusted to maintain a target exercise power of 15W. 3,000 radial projections were acquired continuously for each slice, with 326 samples per spoke and a 0.75 fractional echo: TR/TE/Dz=2.9 ms,1.1ms, 8mm. After binning projections for real-time reconstructions with 20 and 30 spokes per frame (58 and 87 ms temporal resolutions, repectively), images were reconstructed to a matrix of 160x160 for a spatial resolution of 2.25x2.25mm using parallel imaging and compressed sensing (with temporal total variation and spatial wavelet L1-norm penalties) via the BART toolbox7, using coil sensitivity maps determined by ESPIRiT8. The penalty weights were λTV=0.001 and λwavelet=0.001 for the MRXCAT reconstructions and λTV=0.008 and λwavelet=0.007 for the volunteer reconstructions. MRXCAT reconstructions were compared against the ground truth using root mean square error (RMSE), and the volunteer reconstructions were compared visually against cine reconstructions of the same data (20 frames, 75 spokes per frame after respiratory gating from bellows with 50% efficiency, reconstructed with same parameters as real-time reconstructions).RESULTS
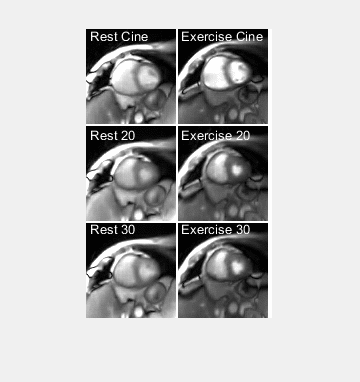
The sampling and reconstruction technique produced MRXCAT images with minimal streaking artifact but with blurring, for both temporal resolutions (Figures 1-2). RMSE was higher for the “exercise” simulation than the rest simulation. RMSE was higher for 20-spoke images than for 30-spoke images at rest, but similar for both temporal resolutions during exercise. The volunteer only had a very slight increase in heart rate during exercise (65 vs. 62 bpm), due to her being on a beta-blocker and to the modest exercise paradigm used as a result of her age and disease. Volunteer images for real-time and cine reconstruction are shown in Figures 3 and 4. Figure 5 plots the intensity along a profile through the left ventricle (LV) at end diastole (ED) during exercise for each of the reconstructions.DISCUSSION
We evaluated the use of radial sampling and compressed sensing at 2 temporal resolutions for real-time exercise CMR in the MRXCAT numerical phantom and a volunteer. This approached generated images with acceptable image quality at both temporal resolutions. The 30-spoke reconstructions resulted in images with lower RMSE in the MRXCAT phantom at “rest”, as increased data reduced undersampling artifact. At “exercise”, however, RMSE was higher in the 30-spoke ED image, but lower in the end systole (ES) image. This likely results from the rapid motion of late diastolic filling which caused intraframe motion-induced blurring, particularly in the 30-spoke reconstruction with a longer temporal resolution. Similar performance was seen in both temporal resolutions in the volunteer, likely because the volunteer’s beta-blocking medication prevented a large heart rate increase in response to exercise, so both temporal resolutions were sufficient to capture the myocardial motion. However, image quality was worse in both real-time exercise images than the corresponding rest images, perhaps due to increased myocardial contractility or bulk motion. The profile through the LV highlights possible marginal benefits of a shorter temporal resolution, with a slightly better match to the cine reconstruction for the 20-spoke vs. 30-spoke image. A limitation is the low HR during exercise of the volunteer. Future work should test this technique in subjects capable to exercise at higher workloads to test the approach at increased heart rates.CONCLUSIONS
Real-time exercise CMR is feasible with radial sampling and compressed sensing at sufficient spatial and temporal resolution to capture myocardial motion. While a longer temporal resolution provides better image quality during rest, shortening the temporal resolution can improve results during exercise by better capturing rapid motion.Acknowledgements
Philip A Corrado is supported by the National Heart, Lung, And Blood Institute of the NIH under Award Number F31HL144020. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We gratefully thank GE Healthcare for MRI research support.References
1. Nagel E, van Rossum AC, Fleck E. Cardiovascular Magnetic Resonance. Steinkopff; 2013.
2. La Gerche A, Claessen G, Van De Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac MRI: A new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. 2013;6:329–338. doi: 10.1161/CIRCIMAGING.112.980037
3. Feng X, Salerno M, Kramer CM, Meyer CH. Non-Cartesian balanced steady-state free precession pulse sequences for real-time cardiac MRI. Magn Reson Med. 2016;75:1546–1555. doi: 10.1002/mrm.25738
4. Zhao B, Haldar JP, Brinegar C, Liang Z-PP. Low rank matrix recovery for real-time cardiac MRI. 2010 7th IEEE Int Symp Biomed Imaging From Nano to Macro, ISBI 2010 - Proc. 2010;996–999. doi: 10.1109/ISBI.2010.5490156
5. Wundrak S, Paul J, Ulrici J, Hell E, Rasche V. A small surrogate for the golden angle in time-resolved radial MRI based on generalized fibonacci sequences. IEEE Trans Med Imaging. 2015;34:1262–1269. doi: 10.1109/TMI.2014.2382572
6. Wissmann L, Santelli C, Segars WP, Kozerke S. MRXCAT: Realistic numerical phantoms for cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2014;16:1–11. doi: 10.1186/s12968-014-0063-3
7. Uecker M, Tamir JI, Ong F, Lustig M. The BART Toolbox for Computational Magnetic Resonance Imaging. Ismrm. 2016;
8. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT--An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014;71. doi: 10.1002/mrm.24751
Figures