0502
Predicting disability from structural and functional coupling in multiple sclerosis1Department of Radiology, Weill Cornell Medicine, New York, NY, United States, 2Judith Jaffe Multiple Sclerosis Center, Weill Cornell Medicine, New York, NY, United States, 3Department of Neurology, Weill Cornell Medicine, New York, NY, United States
Synopsis
The complex relationship between brain’ structural connectivity (SC) and functional connectivity (FC) has not yet been fully quantified. Previous studies have shown that an increased SC-FC coupling is associated with worse cognitive performance and higher disability in people with multiple sclerosis (pwMS). However, no study to date investigated the association of regional SC-FC coupling with disability in MS. We showed that the SC-FC coupling performed a high prediction performance in classifying pwMS by impairment level. Damage to SC, particularly in the right parsorbitalis, thalamus, and parahippocampal and left superior parietal is a hallmark of disability in MS.
Introduction
Multiple Sclerosis (MS) is a chronic disease characterized by inflammatory and demyelinating plaques within the central nervous1. The inflammation, demyelination, and axonal loss in people with MS (pwMS) disrupts the brain’s structural connectome (SC), resulting in alteration of the flow of activation throughout the brain, which can be investigated by analyzing the functional connectome (FC). Even though it has been shown that SC and FC are related, the complex relationship between the two has not yet been fully quantified2,3. It is imperative to use both modalities to understand MS because of the intricate interplay between SC and FC in damage and recovery4. Some previous studies explored the association in SC-FC changes with cognitive impairment in MS5,6, while other studies investigated the relationship between disability and SC or FC alone as well as concatenating both connectomes together7,8. It has been shown that increased SC-FC coupling is associated with worse cognitive performance and higher disability in pwMS6. However, no study to date investigated the association of disability with regional SC-FC coupling considering both amplitude and direction of these two connectome types in MS. Therefore, the aims of this study are to 1)assess the regional differences in SC degree, FC degree, and SC-FC coupling between impaired vs not-impaired pwMS and 2)compare the prediction accuracy of SC and FC degrees, and SC-FC coupling in classifying pwMS by impairment level.Methods
MRI data were acquired on a 3T Siemens scanner with resting-state fMRI (6 min, TR = 2.3 s, 3.75 x 3.75 x 4 mm voxels) and diffusion MRI (55 directions HARDI, b=1000, 1.8 x 1.8 x 2.5 mm voxels). The mean fMRI signal over all voxels in a region was calculated and the mean regional time series correlated between every pair of regions to obtain FC. We performed probabilistic (ifod2) tractography with MRtrix39,10, after which the SIFT2 global filtering algorithm11 was applied. The SC was constructed by taking the sum of the SIFT2 weights of streamlines connecting pairs of regions and dividing SC matrix rows by the volume of that region.Seventy-six MS patients (age: 45.37 ± 11.44 years, 66% female, disease duration: 12.48 ± 7.22 years) were included in our study. Expanded Disability Status Scale (EDSS) was used to assess the disability. 68 had less than EDSS 3 at study baseline. Wilcoxon rank-sum test was applied to compare the regional SC-FC coupling between not-impaired vs impaired groups. Ridge regression was applied to assess the prediction accuracy of the models included 1)SC degree, 2)FC degree, 3)concatenation of SC&FC degrees, and 4) SC-FC coupling. SC and FC were measured between 86 Freesurfer based gray matter regions. SC and FC degree were computed as the sum of the connections from a region to other regions. SC-FC coupling was constructed by calculating the Pearson’s correlation between a row of the SC matrix with the corresponding row of the FC matrix. SMOTE12 function was used to obtain a balanced training dataset during 5-fold cross-validation. The cross-validation was repeated with 100 outer loops. The model performance was assessed with the area under the ROC curve (AUC). Student’s t-test was used to compare the AUC values from different models. Differences were considered significant when p<0.05 after Benjamini-Hochberg (BH)13 correction for multiple comparisons.
Results
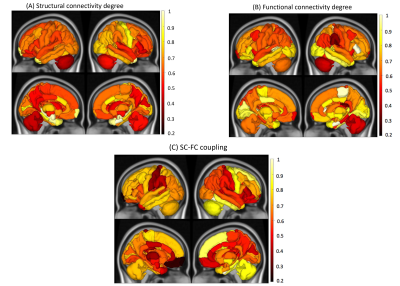
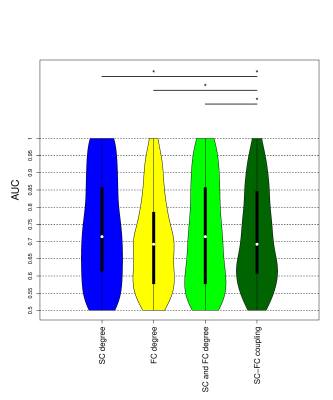
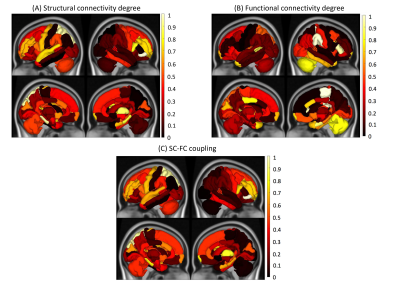
SC degree in the subcortical regions such as bilateral hippocampus, bilateral amygdala, bilateral entorhinal as well as right temporal pole was different between not-impaired vs impaired pwMS (See Figure 1). FC degree in right entorhinal, parstriangularis and paracentral, while SC-FC coupling in the temporal and frontal lobes, primary motor cortex, and right cerebellum was significantly between two impairment groups. However, no significant difference was found after BH correction.All models showed high prediction accuracies with an average AUC >0.70 in classifying pwMS by impairment level. The models that included SC degree and concatenated SC and FC degree outperformed the models that included FC degree and SC-FC coupling (p-value<0.05, corrected) (See Figure 2). There was no significant difference in AUC between the models that included SC degree and concatenated SC&FC degree (p-value=0.22, corrected) as well as between the models included FC degree and SC-FC coupling (p-value=0.23, corrected).
Figure 3 shows that the right parsorbitalis, thalamus, and parahippocampal and left superior parietal were commonly found as the most important regions in the SC degree and SC-FC coupling models, while FC degree in the right paracentral, right supramarginal and right parstriangularis was the most important predictors in classifying pwMS by impairment level.
Discussion
SC-FC coupling in the temporal and frontal lobes, primary motor cortex, and right cerebellum was stronger in the impaired pwMS. SC degree and a concatenation of SC&FC degree better classified the pwMS than other models. These results suggest that damage to SC, particularly in the right parsorbitalis, thalamus, and parahippocampal and left superior parietal is a hallmark of disability in MS. Future work including a higher number of impaired pwMS needs to be performed to validate the results.Conclusion
Impaired pwMS exhibited stronger SC-FC coupling in the subcortical, primary motor, and cerebellum regions as compared to not impaired pwMS. SC degree proved to be more discriminative imaging modality than FC degree and SC-FC coupling in classifying pwMS by impairment level.Acknowledgements
C.T. helped with image post-processing, carried out the statistical analyses and wrote the abstract.K.J. collected the data and performed pre- and post-processing of MRI data.S.G. collected the data and helped interpret results.A.K. designed and supervised the study and collected the data.
The authors declare that they have no competing interest.
This work was supported by the NIH R21 NS104634-01 (A.K.), NIH R01 NS102646-01A1(A.K.), and grant UL1 TR000456-06 (S.G.) from the Weill Cornell Clinical and TranslationalScience Center (CTSC).
References
1. Weinshenker BG, Rice GPA, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. TThe natural history of multiple sclerosis: a geographically based study 3. Multivariate analysis of predictive factors and models of outcome. 1991;114:1045–1056.
2. Abdelnour F, Voss HU, Raj A. Network diffusion accurately models the relationship between structural and functional brain connectivity networks. Neuroimage. 2014;90:335–47.
3. Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2035–2040.
4. Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 2009;
5. Koubiyr I, Deloire M, Besson P, Coupé P, Dulau C, Pelletier J, et al. Longitudinal study of functional brain network reorganization in clinically isolated syndrome. Mult. Scler. J. 2018;
6. Koubiyr I, Deloire M, Brochet B, Besson P, Charré-Morin J, Saubusse A, et al. Structural constraints of functional connectivity drive cognitive impairment in the early stages of multiple sclerosis. Mult. Scler. J. [Internet]. 2020 [cited 2020 Dec 9];Available from: https://doi.org/10.1177/1352458520971807https://doi.org/10.1177/1352458520971807
7. Muthuraman M, Fleischer V, Kolber P, Luessi F, Zipp F, Groppa S. Structural Brain Network Characteristics Can Differentiate CIS from Early RRMS. Front. Neurosci. [Internet]. 2016 [cited 2019 May 2];10:14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26869873
8. Tommasin S, De Giglio L, Ruggieri S, Petsas N, Giannì C, Pozzilli C, et al. Relation between functional connectivity and disability in multiple sclerosis: a non-linear model. J. Neurol. [Internet]. 2018 [cited 2018 Oct 8];1–12. Available from: http://link.springer.com/10.1007/s00415-018-9075-5
9. Tournier J-D, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. [Internet]. 2012 [cited 2020 May 7];22:53–66. Available from: http://doi.wiley.com/10.1002/ima.22005
10. Tournier J-D, , F. Calamante and a. C. Improved probabilistic streamlines tractography by 2 nd order integration over fibre orientation distributions. Ismrm. 2010;88:2010.
11. Smith RE, Tournier J-D, Calamante F, Connelly A. The effects of SIFT on the reproducibility and biological accuracy of the structural connectome. Neuroimage. 2015;104:253–265.
12. Chawla N V., Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. [Internet]. 2002 [cited 2019 May 29];16:321–357. Available from: https://jair.org/index.php/jair/article/view/10302
13. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B [Internet]. 1995 [cited 2020 Aug 23];57:289–300. Available from: https://rss.onlinelibrary.wiley.com/doi/full/10.1111/j.2517-6161.1995.tb02031.x
Figures