0483
Differential Changes in Brain Viscoelastic Properties Observed with MR Elastography in MS and NMOSDs1Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, 2Department of Radiology, Mayo Clinic, Rochester, MN, United States, 3Department of Neurology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Synopsis
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by demyelination, axonal loss and neurodegeneration. Because of overlapping clinical and imaging features, it is a challenge to distinguish MS from Neuromyelitis optica spectrum disorders (NMOSDs) for which the treatment is different. 3D MR Elastography (MRE) is a potential method to evaluate brain tissue damage in autoimmune diseases of the CNS. By measuring the viscoelasticity of the centrum ovale with 3D MRE, we found significantly decreased damping ratio and loss modulus in MS compared with NMOSDs, suggesting possible diagnostic utility for 3D MRE in MS.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by demyelination, axonal loss and neurodegeneration, always leading to neurologic disability1. Neuromyelitis optica spectrum disorders (NMOSDs) were considered a variant of MS for many years, for which the treatment is different. Although diagnosis of NMOSDs has been facilitated by the detection of the immunoglobulin G (IgG) antibody to the astrocyte water channel aquaporin 4 (AQP4-IgG), it is still a challenge to distinguish MS from NMOSDs for overlapping clinical and imaging features, particularly from AQP4-seronegative patients2-4. 3D MR Elastography (MRE) is a potential method to evaluate brain tissue damage in autoimmune diseases of the CNS. Animal models have shown a causal relationship between demyelination and decreased brain stiffness, as well as between inflammation and decreased storage and loss moduli5-6. Reductions of stiffness of the brain parenchyma were observed in both MS and NMOSDs patients7-9. While the differential diagnosis performance of 3D MRE in autoimmune diseases of the CNS is unclear. The aim of this study is to assess characteristic changes in 3D MRE-based measurements of the viscoelastic properties of the centrum ovale in patients with MS and in NMOSDs.Methods
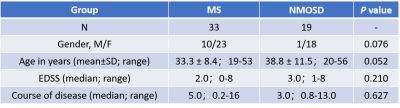
Following ethics committee approval with a waived informed consent requirement, 33 MS patients (according to McDonald’s 2010 criteria) and 19 NMOSDs patients (according to 2015 International Panel for Neuromyelitis Optica Diagnosis criteria) were examined using conventional MRI and MRE scans with 60-Hz vibration frequency. The acquisition parameters for 3D MRE were as follows: TR/TE = 2000/62 ms, FOV = 24×24 cm; acquisition matrix = 96×96; number of excitations = 1; Bandwidth = 250 kHz; slices thickness = 3 mm with gap = 0 mm. Clinical parameters including the expanded disability status scale (EDSS) and disease course (defined as years since diagnosis) were recorded. The success of MRE was defined as visually detectable wave propagation in the whole brain. ROI-based stiffness, storage modulus and loss modulus measurements in the centrum ovale were obtained, and ROIs were drawn with reference to the MR images. The damping ratio (the loss modulus divided by two times the storage modulus) was also calculated. The MRE parameters were analyzed by a two-tailed unpaired t-test, or the Mann-Whitney test if data did not assume a Gaussian distribution. Related parameters were compared to the disease course and EDSS score with Pearson correlation. Statistical significance was defined as P<0.05.Results
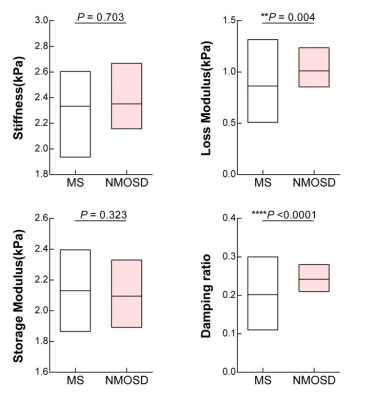
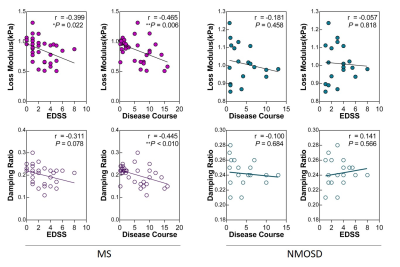
Demographic data and clinical characteristics for the two groups are shown in Table 1. MRE images for 2 subjects are shown in Figure 1. In MS patients, the damping ratio (0.20 ± 0.04 vs. 0.24± 0.02, P<0.0001) and the loss modulus (0.86 ± 0.20 kPa vs. 1.01 ± 0.11 kPa, P=0.004) of the centrum ovale were significantly decreased compared to NMOSDs patients (Figure 2). The correlation analysis showed the negative correlations of the damping ratio with disease course (r=-0.445, P=0.0095) and of the loss modulus with increasing EDSS and disease course (EDSS: r=-0.399, P=0.022; disease course: r=-0.465, P=0.006) in MS patients, but not in NMOSDs (all P>0.05) (Figure 3).Discussion
Our results showed significant decreased damping ratio and loss modulus of the centrum ovale in MS compared with NMOSDs. It has been reported that damping ratio is associated with inflammation in the liver10, so it may be that damping ratio is also a potential noninvasive biomarker to reflect the inflammatory status of the brain that can be used to evaluate therapeutic effects and prognosis. There are negative correlations or trends between these two parameters and clinical information (disease course and EDSS) in MS, but not in NMOSDs, which suggests that brain damage in MS contributes more to EDSS than it does in NMOSDs and MS is more likely to progress with the prolongation of disease course. Due to the limited sample size, larger patient studies are needed to investigate this possibility.Conclusion
Damping ratio and loss modulus in the centrum ovale, one of the most affected sites in MS, were significantly decreased in patients with MS compared with NMOSDs. These two parameters also negatively correlated with disease course in MS, suggesting the potential of MRE to assess clinical severity and therapeutic effects in MS.Acknowledgements
The authors state that this study has received funding by National Natural Science Foundation of China grant 91959118 (JW), Science and Technology Program of Guangzhou, China 201704020016 (JW) and Clinical Research Foundation of the 3rd Affiliated Hospital of Sun Yat-Sen University YHJH201901 (JW).References
1. Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008; 31:247-269.
2. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum antibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004, 364: 2106–12.
3. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015, 85: 177–89.
4. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17: 162-173.
5. Schregel K, Wuerfel E, Garteiser P, et al. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc Natl Acad Sci USA. 2012, 109:6650-6655.
6. Riek K, Millward JM, Hamann I, et al. Magnetic resonance elastography reveals altered brain viscoelasticity in experimental autoimmune encephalomyelitis. Neuroimage Clin. 2012, 1:81-90.
7. Streitberger KJ, Fehlner A, Pache F, et al. Multifrequency magnetic resonance elastography of the brain reveals tissue degeneration in neuromyelitis optica spectrum disorder. Eur Radiol. 2017, 27(5):2206–2215.
8. Wuerfel J, Paul F, Beierbach B, et al. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage. 2010, 49:2520-2525.
9. Streitberger KJ, Sack I, Krefting D, et al. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. Plos One. 2012, 7: e29888.
10. Yin M, Glaser KJ, Manduca A, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology. 2017, 284:694-705.
Figures