0474
Faster regional cerebral blood flow increases in infant heteromodal cortex with 2.5mm3 resolution 3D multi-shot, stack-of-spirals pCASL
Minhui Ouyang1, John Detre2, Chenying Zhao1,3, Samantha Lam1, J. Christopher Edgar1,2, and Hao Huang1,2
1Department of Radiology, The Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, PA, United States
1Department of Radiology, The Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
During early infancy, rapid increases of regional cerebral blood flow (rCBF) supports the metabolic needs for the dramatic maturation of infant brains. In this study, we delineated the developmental pattern of infant’s rCBF from 0-18months, with an optimized 3D multi-shot, stack-of-spirals pCASL sequence for high-resolution rCBF at isotropic 2.5mm. Significant age-related rCBF increases were found during this period. The rCBF growth rates were inhomogeneous across the cortex, with faster maturation rates in the heteromodal association cortex and slower in unimodal sensorimotor cortices. Cortical regions with more rapid rCBF growth were associated with faster microstructural maturation of adjacent white matter.
Purpose
Striking structural and functional brain changes during early infancy 1 are associated with physiological changes to meet the metabolic requirements of these developmental processes. Cerebral blood flow (CBF) has been shown to be tightly coupled with regional metabolism 2,3. Comprehensively delineating the developmental pattern of infant regional CBF (rCBF) may offer insight into the physiological underpinnings of brain maturation during this critical period. In this study, we aimed to elucidate the normative maturational pattern of high-resolution infant rCBF with isotropic 2.5mm, acquired with a 3D multi-shot, stack-of-spirals pseudo-continuous arterial spin labeled (pCASL) sequence optimized for infant brains.Methods
Infant subjects and acquisition of pCASL and multimodal MRI: Twenty-three infants (16 females, age range: 1 to 17 months) were recruited. High-resolution ASL images were acquired with a 3D multi-shot, stack-of-spirals pCASL sequence 4,5 in a 3T Siemens Prisma system. The pCASL MRI parameters were: four-shot acquisition, field of view (FOV) = 192×192 mm2, matrix = 76×76, in-plane resolution = 2.53×2.53 mm2, 48 slices, slice thickness = 2.5 mm, no gap between slices, labeling duration = 1600ms, post labeling delay (PLD) = 1800ms, center of labeling slab located between cervical vertebrae C2 and C3, repetition time = 4s, echo time = 12ms, number of controls/labels = 10 pairs. Phase-contrast (PC) MRI was also acquired to quantify whole-brain CBF 6. In addition, high-resolution T1-weighted images (T1w) with a voxel size of 0.8 mm3 and diffusion MRI (dMRI) with a voxel size of 1.5 mm3 were acquired. rCBF quantification: rCBF were calculated using the single-compartment model 7:$$rCBF=\frac{(6000\cdot\lambda\cdot\triangle{M}\cdot{exp}(\frac{PLD}{T_{1a}}))}{2\alpha\cdot M_b^0\cdot{T_{1a}}\cdot (1-exp(\frac{-LabelDur}{T_{1a}}))} $$, where ΔM is the difference between dynamic-averaged signal intensity in control image and that in the label image; labeling efficiency was assumed to be 0.85, and blood T1 value of arterial blood was assumed to be 1800ms 8, Mb0 values were estimated with the M0 images from the acquisition. Age-related changes in rCBF across cortex: All infants’ rCBF maps were mapped to the JHU atlas space 9. Briefly, infants’ T1w images were first registered to their M0 images in the native CBF space. Then, a 12-parameter affine registration transformed the co-registered T1w image of each infant to the template T1w image in the JHU atlas space, followed by a non-linear transformation. Trajectories of age-related rCBF changes were modeled using linear regression in the cortical atlas regions. White matter (WM) microstructural maturation: dMRI fractional anisotropy (FA) measures were used to explore the maturation of WM microstructural of selected regions of interests (ROIs).
Results
Test-retest reproducibility of rCBF was examined by scanning a healthy young adult twice using the same scanner and identical pCASL protocol (Fig 1A). The intraclass correlation coefficient was 0.96 with the 95% confident intervals [0.95,0.96] (shown in Fig. 1B), indicating excellent reliability of rCBF maps obtained with the 3D multi-shot, stack-of-spirals pCASL perfusion MRI. Fig. 2 shows high-resolution rCBF maps, with densely acquired axial slices, from three representative infants aged 2, 8 and 17 months. RCBF increases during infancy can be clearly seen from the representative maps. The global CBF values obtained from PC MRI of 21 infants demonstrate significant age-related increases (r=0.82, p<2×10-6) shown in Fig. 3A. Specifically, the value of global CBF in infants at 17 months (~66 ml/100g/min) is more than twice of that in infants at 1 month (~30 ml/100g/min). With high-resolution infant rCBF maps, we further examined age-related changes at the regional level. RCBF significantly increased throughout the cortex (Fig. 3B, t(21)>2.83, p<0.01), most prominently in the heteromodal cortex including prefrontal cortex, inferior parietal lobule, lateral temporal cortex and posterior cingulate cortex (PCC). Notably, these cortical regions are known hubs of the default-mode network (i.e., PCC) and frontoparietal network (i.e., prefrontal cortex). Heterogenous rCBF growth rates during infancy were observed across the cortex (Fig. 3C), with faster maturation rates in the heteromodal association cortex (in warm color) and slower maturation rates in unimodal sensorimotor cortices (in cool color). We further explored whether the heterogeneity of rCBF growth was associated with the maturation of adjacent white matter microstructure. Two representative ROIs were placed at the adjacent WM regions of frontal and sensorimotor cortices (left panel of Fig. 4). A faster increase of FA values was found in the WM adjacent to the frontal cortex than sensorimotor cortices (p=0.0036), indicating that cortical regions with more rapid rCBF growth are associated with faster microstructural maturation of the adjacent WM (right panel of Fig. 4).Discussion and conclusion
Using an optimized infant pCASL protocol with isotropic 2.5mm resolution, the present study demonstrated the normative developmental pattern of rCBF during early infancy. RCBF increases rapidly and heterogeneously across the cortex, with faster increases in the heteromodal association cortex, including critical hubs of the default-mode and frontoparietal networks. Cortical regions displaying more pronounced rCBF increases were also characterized by faster maturation of adjacent WM microstructures. The developmental pattern of rCBF may underlie the regionally differentiated metabolic needs that drive increases in blood supply during infant brain maturation. Acquisition and analysis of more infant pCASL data is underway.Acknowledgements
This study is funded by NIH MH092535, MH092535-S1, HD086984 and HD093776.References
- Ouyang M, Dubois J, Yu Q, Mukherjee P, Huang H, 2019. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage, 185: 836-850.
- Fox PT, Raichle ME, 1986. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83(4):1140-1144.
- Gur RC, Ragland JD, Reivich M, Greenberg JH, Alavi A, Gur RE, 2009. Regional differences in the coupling between resting cerebral blood flow and metabolism may indicate action preparedness as a default state. Cerebral Cortex, 19(2): 375-382.
- Chang YV, Vidorreta M, Wang Z, Detre JA, 2017. 3D-Accelerated, Stack-of-Spirals acquisitions and reconstruction of arterial spin labeling MRI. Magn Reson Med 78:1405-1419.
- Vidorreta M, Wang Z, Chang YV, Wolk DA, Ferandez-Seara MA, Detre JA, 2017. Whole-brain background suppressed pCASL MRI with 1D-accelerated 3D RARE stack-of-spirals readout. PLoS one 12(8): e0183762.
- Ouyang M, Liu P, Jeon T, Chalak L, Heyne R, Rollins NK, Licht DJ, Detre JA, Roberts TP, Lu H, Huang, H., 2017. Heterogeneous increases of regional cerebral blood flow during preterm brain development: Preliminary assessment with pseudo-continuous arterial spin labeled perfusion MRI. Neuroimage, 147: 233-242.
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, et al, 2015. Recommended implementationof arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116.
- Varela M, Hajnal JV, Petersen ET, Golay X, Merchant N, Larkman DJ, 2011. A method for rapid in vivo measurement of blood T1. NMR Biomed. 24: 80–88.
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al, 2009. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. Neuroimage, 46(2): 486-499.
Figures
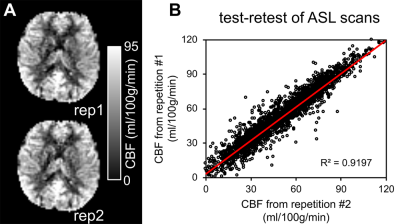
Fig. 1: (A) Reproducible regional
cerebral blood flow (rCBF) maps of a healthy adult from two repetitions of
pseudo-continuous arterial spin labeled (pCASL) scans. (B) the test-retest analysis plot of two pCASL scans.
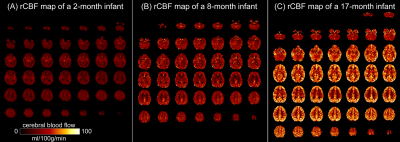
Fig. 2: High-resolution
(2.5x2.5x2.5mm3) regional cerebral blood flow (rCBF) maps acquired
with 3D multi-shot, stack-of-spirals pCASL, from three representative infants
aged 2months (A), 8months (B) and 17months (C).
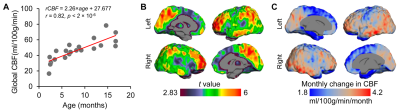
Fig.
3: Main effect of age on cerebral blood flow
(CBF) during infancy. (A) Global CBF increases during infancy. Data points
represent global CBF measured with phase-contrast MRI of each subject. (B) rCBF
increases throughout the cortex, but more prominently in the heteromodal
association cortex. Images thresholded at t(df=21) > 2.83 with p<0.01. (C)
heterogeneous developmental rate of rCBF (ml/100g/min/month) across
cortex during infancy. Slower and faster rCBF growth rates are shown in cool
and warm colors, respectively.
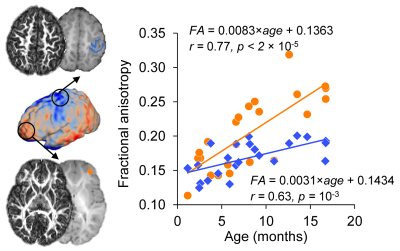
Fig. 4: Cortical regions with more rapid rCBF growth were associated with
faster maturation of the adjacent white matter (WM). Left:
Microstructural maturation was evaluated in adjacent WM to areas of slower and faster
rCBF growth rate. WM maturation was quantified by fractional anisotropy (FA)
measures in sensorimotor (in blue; Top) and frontal (in orange; Bottom) cortices.
ROIs were overlaid on the T1w images with corresponding FA maps displayed to the
side. Right: Scatterplots with linear regressions shows faster
maturation of WM adjacent to frontal than sensorimotor cortices.