0471
phMRI with Simultaneous Measurement of Cerebral Perfusion and Blood-Cerebrospinal Fluid Barrier Function using Interleaved Echo-Time ASL1Centre for Advanced Biomedical Imaging, University College London, London, United Kingdom, 2Neuroradiological Academic Unit, Department of Brain Repair and Rehabilitation, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 3Leonard Wolfson Experimental Neurology Centre, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 4Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom
Synopsis
We employed an interleaved short/long echo-time ASL sequence to better understand the differential response of vessels associated with the blood brain barrier (BBB), and the relatively understudied blood-cerebrospinal fluid barrier (BCSFB) to pharmacological perturbation in the healthy and aged brain. We measured changes in both cortical perfusion and the BCSFB-ASL signal in response CO2, caffeine, and vasopressin. Additionally, we demonstrated a marked decrease in BCSFB reactivity towards vasopressin in the aged vs adult brain. Together, these novel data highlight the value of this translational approach to capture simultaneous and differential pharmacological modulation of vessel tone at the BBB and BCSFB.
Introduction
The blood-brain-barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) mediate the complex interplay between blood and the brain. Non-invasive CBF measurements obtained using arterial spin labelling (ASL) MRI provide a surrogate measure of the functionality of the BBB to, for example, facilitate a constant supply of oxygen and nutrients from the blood to the highly metabolically active brain cells. We have recently developed an ASL-MRI technique for the non-invasive assessment of BCSFB function: BCSFB-ASL uses an ultra-long echo time to quantify the rate of BCSFB-mediated delivery of endogenous arterial blood water to ventricular cerebrospinal fluid (CSF)1. Importantly, the technique allows repeated measures with high temporal resolution which enables dynamic capture of the BCSFB’s functional response to drugs for the first time.By alternating the ultra-long and short TEs used for BCSFB- and traditional-ASL, respectively, and keeping all other parameters constant, we are able to simultaneously capture two distinct physiological responses to a single drug dose or ‘challenge’: i) parenchymal perfusion; ii) rates of BCSFB-mediated labelled arterial blood water delivery to the CSF (a surrogate, non-invasive, measure of BCSFB function). This approach provides an efficient means to better understand the differential response of vessels associated with the BBB and BCSFB to pharmacological perturbation in the healthy and aged/diseased brain.
Methods
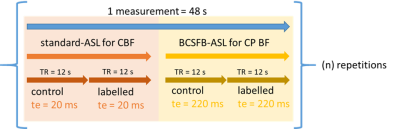
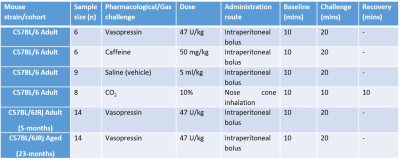
We first applied the interleaved-TE ASL method in the anaesthetized mouse brain (n=6) at 9.4T (Agilent), to investigate the effects of several selected challenges on vessels perfusing the choroid plexus and the cortex: vasopressin, CO2, and caffeine (further details: Table 1). The interleaved-TE ASL protocol (Figure 3) utilized a FAIR-EPI labelling/readout with parameters: TI = 4000ms, TR = 12000ms, TE = 20ms and 220ms for traditional- and BCSFB-ASL, respectively, single 2.4mm slice centred on lateral ventricles, matrix size: 32x32, FOV: 20mm x 20mm.We investigated the mechanistic link between BCSFB function and vasopressin in the aged brain by applying the interleaved-TE ASL technique to capture the phMRI response to vasopressin in a cohort of aged (23-months, n=14) and adult mice (5-months, n=14).
For each subject, a single ROI drawn across the cortex/lateral ventricles provided the mean average signal across the voxels for each slice-selective and non-selective image pair. For each image pair, the slice-selective intensity (ML) had its corresponding non-selective intensity (MC) subtracted to give a ΔM value, which was then divided by its corresponding Mc. In a subject-wise manner, ΔM/MC values were divided by the mean ΔM/MC value for the 10-minute baseline, providing a measure of relative, baseline-normalised perfusion (short TE) and BCSFB-ASL signal (ultra-long TE respectively).
Paired, 2-tailed t-tests were conducted when comparing challenge-responses to baseline. Unpaired, 2-tailed t-tests were conducted to compare adult and aged cohorts.
Results
Administration of saline vehicle revealed no significant changes in cortical perfusion or the BCSFB-ASL signal. As expected, hypercapnia induced increases in relative cortical perfusion (21%, p=0.0038, Figure 1 a-b). A similar increase in the BCSFB-ASL signal from baseline was also observed (21%, p=0.031, Figure 1c-d).A marked vasopressin-induced decrease in the BCSFB-ASL signal was observed (46%, p=0.0004, figures 1g-h). However, the 15% increase in cortical perfusion only bordered significance (p=0.055, Figure 1e-f)
Caffeine stimulated significant decreases in the BCSFB-ASL signal (41% average, p=0.0004, Figure 1k-l). Changes in cortical perfusion were not observed (p=0.25, Figure 1i- j).
Vasopressin induced a marked decrease in the BCSFB-ASL signal in the adult cohort, averaging 27% (Figure 2a-b, p=0.00004). In contrast, aged mice displayed an average 0.7% increase (n.s., p=0.93). This drastic impairment in the response of aged mice vs their adult counterparts was statistically significant (p=0.013, Figure 2b).
Discussion
Here we present a method to capture dynamic responses of the BCSFB to pharmacological modulation. By employing an interleaved-TE ASL sequence, we are also able to measure simultaneous dynamic changes in cortical perfusion.Dampened dilatory responses to CO2 have been implicated in many pathological conditions affecting the brain microvasculature, such as cognitive decline in ageing and dementia2–5. Our results indicate a significant, CO2-driven increase in CP perfusion of a similar magnitude to that observed in the cortex.
Measurements of caffeine-induced CBF changes in humans and rat models have shown CBF decreases6,7. We provide dynamic data assessing the immediate effects of caffeine in the mouse brain for the first time, observing marked decreases only in the BCSFB-ASL signal.
It has become increasingly evident that the-BCSFB undergoes numerous morphological and functional changes within the ageing brain8–10, with wide-ranging evidence that an increased levels of endogenous vasopressin may underlie decline11–15. The observed impairment to a vasopressin challenge observed in the aged brain highlights the potential for novel measurements of BCSFB reactivity to provide upstream biomarkers of age-related pathological processes.
Conclusion
In conclusion, our results illustrate the value of an interleaved-TE ASL MRI approach to quantify pharmacologically-induced changes to vessels associated with the BBB in the cortex and the BCSFB in the choroid plexus. The marked impairment of the response to a vasopressin challenge within the aged mice highlight the capability of such measurements to be utilised for probing altered functionality and pathophysiology in the aged or diseased brain, providing a potential novel biomarker of age-related cognitive decline.Acknowledgements
No acknowledgement found.References
1. Evans, P. G. et al. Non-Invasive MRI of Blood–Cerebrospinal Fluid Barrier Function. Nat. Commun. 11, 1–11 (2020).
2. Zhou, Y., Rodgers, Z. B. & Kuo, A. H. Cerebrovascular reactivity measured with arterial spin labeling and blood oxygen level dependent techniques. Magn. Reson. Imaging 33, 566–576 (2015).
3. Suri, S. et al. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimer’s Dement. 11, 648-657.e1 (2015).
4. Hurford, R. et al. MRI-visible perivascular spaces: Relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J. Neurol. Neurosurg. Psychiatry 85, 522–525 (2014).
5. Kastrup, A., Dichgans, J., Niemeier, M. & Schabet, M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke 29, 1311–1314 (1998).
6. Vidyasagar, R., Greyling, A., Draijer, R., Corfield, D. R. & Parkes, L. M. The effect of black tea and caffeine on regional cerebral blood flow measured with arterial spin labeling. J. Cereb. Blood Flow Metab. 33, 963–968 (2013).
7. Nehlig, A., de Vasconcelos, A. P., Dumont, I. & Boyet, S. Effects of caffeine, L-phenylisopropyladenosine and their combination on local cerebral blood flow in the rat. Eur. J. Pharmacol. 179, 271–280 (1990).
8. Vandenbroucke, R. E. A hidden epithelial barrier in the brain with a central role in regulating brain homeostasis: Implications for aging. in Annals of the American Thoracic Society vol. 13 S407–S410 (American Thoracic Society, 2016).
9. Balusu, S., Brkic, M., Libert, C. & Vandenbroucke, R. E. The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: More than just a barrier. Neural Regeneration Research vol. 11 534–537 (2016).
10. Serot, J. M., Foliguet, B., Béné, M. C. & Faure, G. C. Choroid plexus and ageing in rats: A morphometric and ultrastructural study. Eur. J. Neurosci. 14, 794–798 (2001).
11. Frolkis, V. V., Kvitnitskaya-Ryzhova, T. Y. & Dubiley, T. A. Vasopressin, hypothalamo-neurohypophyseal system and aging. Arch. Gerontol. Geriatr. 29, 193–214 (2000).
12. Liszczak, T. M., Foley, L. & Black, P. M. L. Arginine vasopressin causes morphological changes suggestive of fluid transport in rat choroid plexus epithelium. Cell Tissue Res. 246, 379–385 (1986).
13. Dohrmann, G. J. Dark and light epithelial cells in the choroid plexus of mammals. J. Ultrasructure Res. 32, 268–273 (1970).
14. Johanson, C. E. et al. AVP V1 receptor-mediated decrease in Cl- efflux and increase in dark cell number in choroid plexus epithelium. Am. J. Physiol. - Cell Physiol. 276, (1999).
15. Sturrock, R. R. A morphological study of the development of the mouse choroid plexus. J. Anat. 129, 777–93 (1979).
Figures

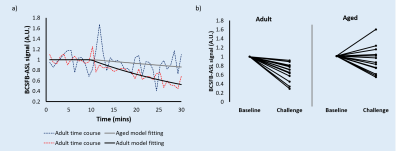
Figure 2 - Adult (n = 14) vs aged (n = 14) response to vasopressin.
a) Relative BCSFB-ASL signal obtained using Interleaved-TE ASL, showing both adult and aged averaged time courses (vasopressin administration at 10 mins).
b) Adult (n = 14) vs aged (n = 14) response to vasopressin, individual animal averages (baseline and post-vasopressin challenge).