0463
Unsupervised Correction of Sub-TR Physiological Noise using Phase and Magnitude fMRI data1High-Field MR Centre, Medical University of Vienna, Vienna, Austria, 2Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 3Department of Neurology, Medical University of Graz, Graz, Austria, 4Centre for Advanced Imaging, University of Queensland, Queensland, Australia
Synopsis
External physiological recordings can be used to filter out cardiac and respiration fluctuations in fMRI data but these can be unreliable. We propose an unsupervised method which derives physiological noise regressors, including cardiac fluctuations with a period much less than the volume TR, from phase and magnitude fMRI data. We compare its efficacy with a correction method which uses external recordings (RETROICOR), and a rival physiological data-free method (PESTICA).
Introduction
Physiological noise from cardiac and respiratory fluctuations limits the achievable tSNR(1) in fMRI, reducing the sensitivity to signals of neuronal origin. The most widely used method to reduce physiological noise, RETROICOR(2), requires measurements of cardiac and respiration signals recorded with a Physiological Monitoring Unit (PMU) to generate regressors which are included in the General Linear Model. These tertiary measurements increase the time overhead in subject handling and are unreliable, motivating approaches which identify physiological signals from the fMRI data directly. One of the most successful data-driven approach, the ICA-based method PESTICA(3; 4), requires user interaction, however.We have developed an unsupervised method PREPAIR (Physiological Regressor Estimation from Phase and mAgnItude, sub-tR) for identifying physiological fluctuations in fMRI data using both magnitude and phase data, given the sensitivity of phase to changes in B0(5;6). To recover cardiac fluctuations, we utilized information in each slice to be able to capture all physiological fluctuations with a period longer than twice the slice-TR, rather than the conventional limit of twice the volume-TR. PREPAIR generates regressors from magnitude and phase and identifies that which best describes respiratory and cardiac signals. We compared our method with RETROICOR, which uses physiological recordings, and PESTICA.
Methods
Whole brain axial MRI data from nine healthy participants were acquired with a Siemens PRISMA 3T using a multiband EPI sequence(7) at various TRs (700;1020;1520 and 2000 ms) and multiband factors MB (8;4;2 and 1 respectively). Each run was preceded by a low-resolution dual-echo gradient-echo prescan for coil-combination with ASPIRE(8; 9).The steps in generating a magnitude and a phase time-series for physiological noise extraction were as follows:
- Phase data were unwrapped with ROMEO(10).
- Voxels in which the mean of the magnitude was less than the 98th percentile of the values were excluded.
- The magnitude and phase in each slice were averaged (using weighted mean for phase).
- The difference between slices in the first volume was removed from slice time-series(11).
- All slices were reordered according to their acquisition order to obtain a magnitude and a phase time-series sampled at N/(TRxMB) (with N the number of slices), faster than the conventional 1/TR.
- Cardiac and respiratory waveforms were prefiltered from the magnitude and phase time-series within [48 – 96] bpm and [9 – 30] bpm respectively before automatically centring the identified fundamental frequencies in windows of ± 10 bpm and ±5 bpm around them respectively.
- Magnitude- and phase-derived physiological regressors were computed considering a 2nd order Fourier expansion.
- The number of voxels with a significant improvement after respiratory and cardiac fluctuations removal was estimated to determine whether the magnitude- or phase-derived regressors better described physiological fluctuations in the data.
Results
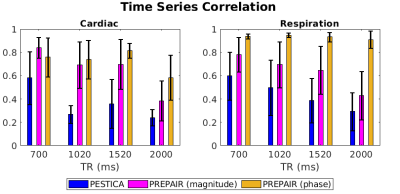
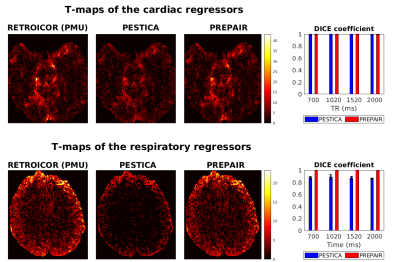
Figure 1 illustrates, for protocols with different TR (x-axis), the correlation between the RETROICOR(PMU), the PESTICA (blue), the PREPAIR-phase (orange) and the PREPAIR-magnitude (magenta) cardiac and respiratory waveforms. PREPAIR correlates better with the RETROICOR(PMU) data than PESTICA, especially the phase.Figure 2 shows a comparison of voxels corrected with cardiac and respiratory regressors obtained with each method. For the cardiac regressors there is good agreement with the RETROICOR(PMU) correction (left) and both PESTICA (centre) and PREPAIR ( right) - evidenced by similar DICE values across subjects. For respiratory regressors, PREPAIR performs better than PESTICA.
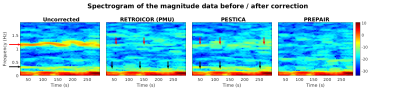
Figure 3 shows the frequency distribution of the magnitude data in average sliding windows of 45s (or spectrograms) in i) uncorrected magnitude, ii) RETROICOR(PMU)-corrected, iii) PREPAIR-corrected and iv) PESTICA-corrected magnitude. Cardiac and respiration frequencies (red and black horizontal arrows, respectively) are completely removed with PREPAIR. A small amount of cardiac and respiratory signal (vertical red and black arrows, respectively) remains in the RETROICOR(PMU)-corrected data, and with a higher amount in PESTICA-corrected data.
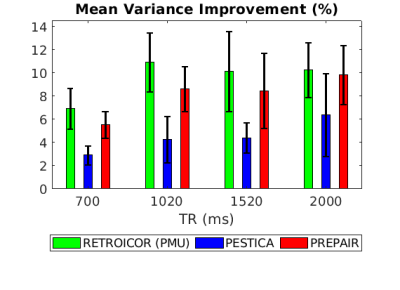
The average image variance improvement is represented in Fig. 4 for all TRs. Including errors bars, PREPAIR (red) can perform as well as the RETROICOR(PMU) (green) and outperforms in all cases PESTICA (blue).
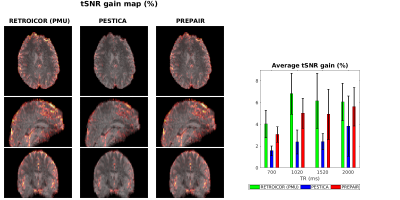
In Fig. 5 representing the tSNR gain after correction, we see that this gain is more significant with PREPAIR (right) than with PESTICA (centre) compared to the RETROICOR(PMU) (left). In average, the tSNR gain across subjects (histograms, right panel) shows that PREPAIR reaches comparable tSNR improvement (up to 8%) as the RETROICOR(PMU) and performs better than PESTICA in denoising voxels.
Discussion
Our method for the identification of physiological fluctuations in fMRI data, PREPAIR, shows that reliable cardiac and respiratory signals can be extracted from magnitude and phase data by reordering slices acquired at the volume TR into a time-series sampled at the slice TR. The main advantage of PREPAIR is that no additional measurements nor user interaction in the physiological regressor estimation are required. A further improvement, which would increase the efficiency of PREPAIR is the removal of sidebands appearing during the slice reordering that can create undesirable frequencies close to the cardiac or respiratory fundamental.Conclusion
We have demonstrated that physiological signals can be identified in phase and magnitude fMRI data, even if their frequency is much higher than 2/TR-vol. Our unsupervised method - PREPAIR - significantly reduces the variance and increase tSNR in voxels close to areas affected by cardiac and respiratory fluctuations.Acknowledgements
This study was funded by the Austrian Science Fund project FWF31452. SDR is supported by Marie Sklodowska-Curie Action MS-fMRI-QSM 794298.References
1. Triantafyllou C, Polimeni JR, Wald LL. Physiological noise and signal-to-noise ratio in fMRI with multi-channel array coils. Neuroimage. 2011;55(2):597-606.
2. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2004;44:162-167.
3. Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. Neuroimage. 2007;37:1286-1300.
4. Beall EB. Adaptive cyclic physiologic noise modeling and correction in functional MRI. Journal of Neuroscience. 2010;187:216-228.
5. Hagberg GE, Bianciardi M, Brainovich V, Cassarà AM, Maraviglia B. The effect of physiological noise in phase functional magnetic resonance imaging: from blood oxygen level-dependent effects to direct detection of neuronal currents. Magn Reson Imaging. 2008;26(7):1026-40.
6. Zahneisen B, Assländer J, Le Van P, Hugger T, Reisert M, Ernst T, Hennig J. Quantification and correction of respiration induced dynamic field map changes in fMRI using 3D single shot techniques. Magn Reson Imaging. 2014;71:1093-1102.
7. Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Uurbil K. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 2013;83(0):991-1001.
8. Eckstein K, Dymerska B, Bachrata B, Bogner W, Poljan K, Trattnig S, Robinson SD.Computationally Efficient Combination of Multi-channel Phase Data From Multi-echo Acquisitions (ASPIRE). Magn Reson Med. 2018;79(6):2996-3006.
9. Bachrata B, Eckstein K, Trattnig S, Robinson SD. Considerations in quantitative susceptibility mapping using echo-planar imaging. Proceeding ISMRM. 2018;4996.
10. Robinson SD, Eckstein K, Trattnig S, Shmueli K, Dymerska B. Rapid Opensource Minimum spanning treE algOrithm for phase unwrapping. Proceeding ISMRM. 2020;3659.
11. Bancelin D, Lima Cardoso P, Bachrata B, Ehrmann A, Trattnig S, Robinson SD. Correcting respiratory-related noise in brainstem fMRI using regressors derived from phase data. Proceeding ISMRM. 2020;4007.
12. Kasper L, Bollman S, Diaconescu AO, Hutton C, Heinzle J, Iglesias S, Hauser TU Sebold M, Manjaly ZM, Pruessmann K, Stephan KE. The PhysIO Toolbox for Modeling Physiological Noise in fMRI Data. J Neurosci Methods. 2017;276:56-7
Figures