0462
A Paradigm Change in Functional Brain Mapping: Suppressing the Thermal Noise in fMRI1CMRR, University of Minnesota, minneapolis, MN, United States, 2Department of Neurosurgery, University Of Minnesota, minneapolis, MN, United States, 3University Of Maastricht, Maastricht, Netherlands
Synopsis
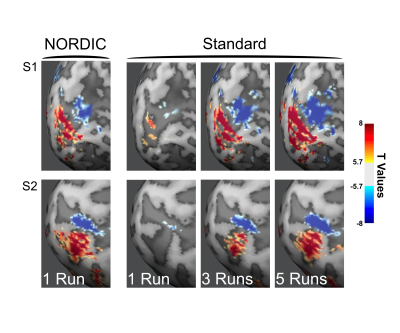
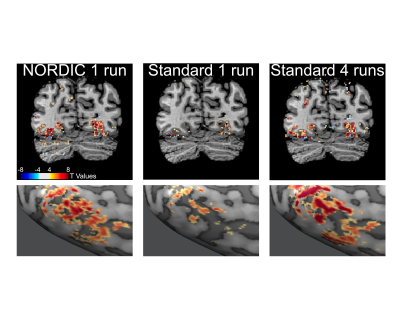
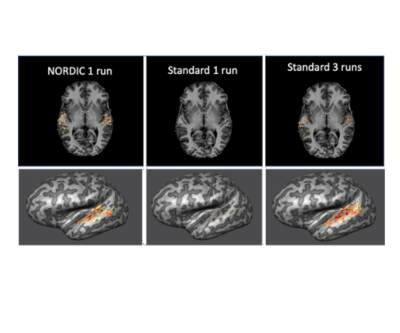
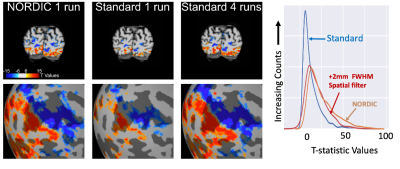
Functional imaging with the BOLD contrast is an essential tool to investigate human brain functions, however the contribution of thermal noise compromises the signal, particularly at higher resolutions. A recently developed technique, NORDIC, aims to suppress this noise source. Here we show that NORDIC produces data with substantially higher signal-to-noise and functional contrast to noise ratios, resulting in improved detection of functional activation with no change in spatial precision. One run of denoised data is equivalent to combining 3 to 5 runs of conventional data. These effects create new opportunities for functional neuroimaging, while reducing participant or patient burden.
Introduction
Functional Magnetic Resonance Imaging (fMRI) (1-3) based on Blood Oxygenation Level Dependent (BOLD) contrast is an indispensable tool employed for investigating human brain activity and functional connectivity.In view of the vast spatial scale over which the brain is organized and operates, it was evident since its inception that the spatiotemporal resolution and functional contrast-to-noise ratio (fCNR) of fMRI had to be significantly improved. This challenge motivated the introduction and the subsequent development of ultrahigh magnetic field (UHF) of 7 Tesla (7T) (4) to increase both the intrinsic signal-to-noise ratio (SNR) as well as the magnitude and the spatial accuracy (relative to the territory of neuronal activity) of BOLD based functional images. This development ultimately led to successful submillimeter resolution fMRI mapping of cortical columns and layers, and other fine-scale organizations (5,6,4), providing the unique opportunity to study mesoscopic scale neuronal organizations in the human brain for the first time. However, the SNR and fCNR of fMRI data in general remain quite low, especially for high resolution functional images targeting mesoscopic neuronal organizations. This is a major limitation to high resolution fMRI applications as well as the utility and ultimate impact of such data.In this work, we tackle the SNR and fCNR limitation in fMRI using a denoising technique, Noise Reduction with Distribution Corrected (NORDIC) PCA (7), which operates on repetitively acquired imaging data like fMRI and diffusion imaging used for tractography (dMRI), and removes all components which cannot be distinguished from zero-mean Gaussian distributed noise. As such, it specifically targets the suppression of thermal noise inherent in the MR measurement. This is the dominant noise source in submillimeter resolution fMRI data at 7T (e.g. obtained with ~0.8 mm isotropic voxel dimensions), but also is a significant contributor to fluctuations in an fMRI time series even in 3Tesla studies conducted with voxel volumes equivalent to 1 to 3 mm isotropic voxels dimensions (8).Methods
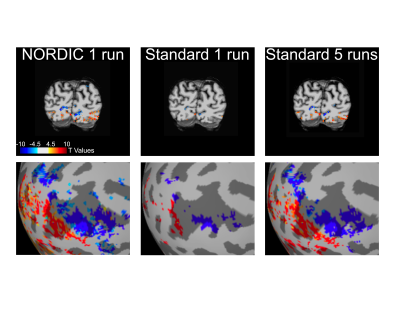
We implemented NORDIC denoising on a variety of widely used fMRI acquisition protocols (such as the Human Connectome Project (HCP) protocols), varying in spatio-temporal resolutions, experimental paradigms and field strengths. Specifically, we acquired 5 task-based fMRI data sets with a variety of experimental designs. These included a block design (12 seconds on/off) with counterphase 6Hz flickering checkerboards implemented at 7T (2D GE; 0.8 mm iso voxels; iPAT 3; MB2; TR 1350ms; 6/8 PF – Figure 1) and 3T (2 mm iso voxels; TR 800ms; MB: 8 – i.e. the 3T HCP protocol - Figure 4 and 1.2 mm iso voxels; TR 2100ms; MB: 4; iPAT 2 – Figure 5); a 7T fast event-related design (2 seconds on/off) with face stimuli (2D GE, 1.6 mm iso voxels; TR 1000 ms; MB 5; iPAT 2 – i.e. the 7T HCP protocols – Figure 2); and a 7T auditory slow event-related paradigm (0.8 mm iso voxels; TR 1600 ms; MB 2; iPAT 3 – Figure 3), with simple sounds presented for 1 seconds with a 10 seconds inter-trial interval. We evaluate NORDIC performance by comparing it to standard reconstruction, assessing differences in SNR and function spatial precision. Multiple repeated functional data sets (runs) were obtained for each protocol so as to compare NORDIC performance on a single run with concatenated multiple runs of the standard constructed data.Results
Regardless of field strength, experimental paradigm and acquisition protocol, NORDIC reconstructed data crucially did not compromise the spatial precision of functional measurements and did not blur the images, but led to significantly (p<0.05 corrected) larger tSNR and t-values associated with BOLD responses in relevant portions of the cortex. Using NORDIC, we demonstrate that, with a single run of data, we are able to achieve activation that is comparable to averaging 3 to 5 runs with standard reconstructions. We further show no meaningful differences (p>0.05) in percent signal changes of BOLD responses, but a substantially higher (p<0.05 corrected) accuracy in the estimation of these response amplitudes for NORDIC reconstructed images. Using point-spread functional measurements and spatial autocorrelation analyses, we demonstrate no blurring of the images following NORDIC denoising compared to scanner reconstructed images.Conclusion
Our data show that NORDIC denoising removes thermal noise inherent in the MR detection process and markedly improves some of the most fundamental metrics of functional activation detection, while crucially preserving spatial and functional precision. We demonstrate its efficacy for 7T functional mapping at high spatial resolution, typical of the rapidly increasing number of studies targeting mesoscopic scale cortical organizations in the human brain. We also show that the approach is applicable to and significantly improves more conventional studies conducted at 7T and 3T, with ≳1 mm resolution. Importantly, NORDIC does not target structured, non-white noise; as such it is complementary to, and in fact beneficial to, denoising algorithms that primarily focus on suppressing such structured noise in fMRI data. The cumulative gains that can be realized by employing NORDIC as well removal of structured noise will enable transformative improvements in fMRI, permitting more precise quantification of functional responses, robust studies of mesoscopic scale organizations, faster acquisitions rates, and/or significantly shorter scan times.Acknowledgements
This research was supported by NIH grants U01EB025144 (K.U.), P41 EB027061 (K.U.), P30 NS076408 (K.U.) and RF1 MH116978 (E.Y.), NSF CAREER CCF-1651825 (M.A.)References
1. Bandettini, P. A., Wong, E. C., Hinks, R. S., Tikofsky, R. S. & Hyde, J. S. Time course EPI of human brain function during task activation. (1992) Magn Reson Med 25, 390-397.
2. Kwong, K. K., Belliveau, J. W., Chesler, D. A., Goldberg, I. E., Weisskoff, R. M., Poncelet, B. P., Kennedy, D. N., Hoppel, B. E., Cohen, M. S., Turner, R., Cheng, H.-M., Brady, T. J. & Rosen, B. R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. (1992) Proc Natl Acad Sci U S A 89, 5675-5679.
3. Ogawa, S., Tank, D. W., Menon, R., Ellermann, J. M., Kim, S. G., Merkle, H. & Ugurbil, K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. (1992) Proc Natl Acad Sci U S A 89, 5951-5955.
4. Ugurbil, K. Imaging at ultrahigh magnetic fields: History, challenges, and solutions. (2018) Neuroimage 168, 7-32.
5. De Martino, F., Yacoub, E., Kemper, V., Moerel, M., Uludag, K., De Weerd, P., Ugurbil, K., Goebel, R. & Formisano, E. The impact of ultra-high field MRI on cognitive and computational neuroimaging. (2018) Neuroimage 168, 366-382.
6. Dumoulin, S. O., Fracasso, A., van der Zwaag, W., Siero, J. C. W. & Petridou, N. Ultra-high field MRI: Advancing systems neuroscience towards mesoscopic human brain function. (2018) Neuroimage 168, 345-357.
7. Moeller, S., Pishardy, P. K., Ramanna, S., Lenglet, C., Wu, X., Yacoub, E., Ugurbil, K. & Akcakaya, M. NOise Reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. (2020) bioRxiv Published on line before Print; DOI: 10.1101/2020.08.25.267062, 2020.2008.2025.267062.
8. Triantafyllou, C., Hoge, R. D., Krueger, G., Wiggins, C. J., Potthast, A., Wiggins, G. C. & Wald, L. L. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. (2005) Neuroimage 26, 243-250.
Figures