0450
Deep-learning based super-resolution reconstruction for 3D isotropic coronary MR angiography in a one-minute scan
Thomas Küstner1,2, Alina Psenicny1, Camila Munoz1, Niccolo Fuin3, Aurelien Bustin4, Haikun Qi1, Radhouene Neji1,5, Karl P Kunze1,5, Reza Hajhosseiny1, Claudia Prieto1, and René M Botnar1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Radiology, Medical Image and Data Analysis (MIDAS), University Hospital of Tübingen, Tübingen, Germany, 3Ixico, London, United Kingdom, 4IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux, INSERM, Centre de recherche Cardio-Thoracuique de Bordeaux, Bordeaux, France, 5MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Radiology, Medical Image and Data Analysis (MIDAS), University Hospital of Tübingen, Tübingen, Germany, 3Ixico, London, United Kingdom, 4IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux, INSERM, Centre de recherche Cardio-Thoracuique de Bordeaux, Bordeaux, France, 5MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
3D whole‐heart coronary MR angiography (CMRA) has shown significant potential for the diagnosis of coronary artery disease. Undersampled motion corrected reconstruction approaches have enabled free-breathing isotropic 3D CMRA in ~5-10min scan time. However, spatial resolution is still limited compared to coronary CT angiography and scan time remains relatively long. In this work, we propose a deep-learning based super-resolution (SR) framework, combined with non-rigid respiratory motion compensation (SR-CMRA), to shorten the acquisition time to <1min. A 16-fold increase in spatial resolution is achieved by reconstructing a high-resolution CMRA (1.2mm3) from a low-resolution acquisition (1.2x4.8x4.8mm3, 50s scan).
Introduction
3D whole‐heart coronary MR angiography (CMRA) has shown significant potential for the diagnosis of coronary artery disease (CAD). The acquisition of 3D whole-heart CMRA remains lengthy, as high-resolution data needs to be acquired for quantification of luminal stenosis. Scan time reduction has been previously achieved by combining undersampling with respiratory non-rigid motion correction enabling free-breathing 3D isotropic CMRA within ~10min scan time1,2. Recently, deep learning-based undersampling reconstruction methods have been proposed to shorten reconstruction time and enabling high-resolution scans in ~7min predictable scan time3. However, spatial resolution is still limited compared to coronary CT angiography (~0.6mm3) and scan time remains relatively long.An alternative approach for accelerating the image acquisition while simultaneously increasing spatial resolution exploits deep learning-based super-resolution4. Images are acquired in low-resolution (with or without imaging acceleration) and retrospectively reconstructed to the high-resolution target in a single image super-resolution setting. Different deep learning strategies have been employed to utilize spatio-temporal information sharing on a patch- or image-level5-8.
In this work, we propose a deep learning-based super-resolution framework, combined with non-rigid respiratory motion correction (SR-CMRA), to further shorten the acquisition time. Furthermore, in contrast to previous methods, we propose a deep-learning generative adversarial super-resolution network that generalizes to different input resolutions and can operate on an image or patch-level enabling flexible integration into either image or patch-based reconstruction methods. In this study, we analyse the proposed method for a 16-fold increase in spatial resolution by reconstructing a high-resolution (HR) CMRA (0.9mm3 or 1.2mm3) dataset from low-resolution (LR) acquisitions (1.2x3.6x3.6mm3 or 1.2x4.8x4.8mm3; superior-inferior x left-right x anterior-posterior). The supervised SR-CMRA was trained in a cohort of 47 CMRA patients with suspected CAD whereas testing was performed in retrospectively downsampled images (LR: 1.2x3.6x3.6mm3 and 1.2x4.8x4.8mm3) of 5 patients to quantify performance and in 5 prospectively acquired patients with a LR CMRA acquisition (LR: 1.2x4.8x4.8mm3) in less than one minute scan time.
Methods
The proposed SR framework is depicted in Fig.1. ECG-triggered 3D whole-heart Cartesian bSSFP CMRA was acquired under free-breathing in coronal orientation in patients with suspected CAD using a prototype sequence at 1.5T (Aera, Siemens Healthcare, Erlangen Germany). Imaging parameters are stated in2. Briefly, data was acquired with a variable-density CASPR sampling in ~7min (2.5x acceleration) for an isotropic resolution of 1.2mm3 and in ~8min (5x acceleration) for a resolution of 0.9mm3. 2D image navigators were used for retrospective beat-to-beat translational respiratory motion correction and respiratory binning of the 3D CMRA data. Images were reconstructed with a non-rigid motion-compensated PROST2 serving then as HR target. LR images were acquired with matching parameters in ~50s (1x fully sampled) for a resolution of 1.2x4.8x4.8mm3.We propose a generative adversarial network which consists of two cascaded Enhanced Deep Residual Network for SR9 generator, a trainable discriminator10 and a pre-trained perceptual loss network (VGG-1611). The EDSR is built up of two stages each performing a 2-fold upsampling in each direction in 8 consecutive residual blocks with 3x3 convolution filters of stride 1 and 64 kernels. The patch discriminator network is built as a convolutional neural network with dyadic kernel increase and alternating stride of 1 and 2. The input to the network is the LR-CMRA (1.2x3.6x3.6mm3 or 1.2x4.8x4.8mm3) whereas the output is the corresponding isotropic SR-CMRA image (0.9mm3 or 1.2mm3). The input can be either an axial 2D patch with a size in the range of 10% - 90% of the full image slice or an axial 2D image slice (i.e. a single 2D patch of 100% size). The network is trained in a supervised manner on 460,000 axial pairs of HR-CMRA (0.9mm3 and 1.2mm3) and retrospectively downsampled LR images from 47 patients. Retrospectively simulating a 25% phase and slice resolution provides the LR training input (readout resolution was not affected). The perceptual loss network and the discriminator were pre-trained on a cohort of 50 patients with suspected CAD scanned by CT coronary angiography. In total, the network consists of ~6.3 million trainable parameters optimized under mean absolute error (MAE), structural similarity index (SSIM), adversarial and perceptual loss by an Adam optimizer (batch size=16, 60 epochs). Testing was done on 5 patients (retrospective cohort; not seen in training) with retrospectively downsampled LR images from HR target (0.9mm3 or 1.2mm3) and in 5 patients (prospective cohort; not seen in training) with prospectively acquired LR-CMRA of 1.2x4.8x4.8mm3 and with a separate HR acquisition (1.2mm3).
Results and Discussion
The LR input, HR acquisition and SR-CMRA output of one prospectively acquired test patient and one retrospectively downsampled test patient are shown in Fig. 2 and 3, respectively. The proposed network generalized for varying imaging resolutions and for patch- or image-input as shown in Fig. 4. Qualitatively and quantitatively the proposed SR-CMRA showed significant improvement in edge sharpness and vessel delineation as well as depiction of coronary arteries with respect to LR-CMRA and comparable image quality to the HR-CMRA (Fig. 5). The proposed SR-CMRA required ~186hrs for training but only ~3s for reconstruction.Conclusion
In conclusion, the proposed SR-CMRA has the potential to provide a 16-fold increase in resolution with comparable image quality to the HR-CMRA image while reducing the predictable scan time to <1min.Acknowledgements
This work was supported by EPSRC (EP/P001009, EP/P032311/1, EP/P007619/1), BHF programme grant RG/20/1/34802 and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Cruz et al. MRM 2017;77(5).2. Bustin et al. JCMR 2020;22(1).
3. Fuin et al. Magnetic Resonance Imaging 2020;70.
4. Lundervold et al. Zeitschrift für Medizinische Physik 2019;29(2).
5. Xie et al. ISMRM 2018. p1050.
6. Lin et al. arXiv preprint arXiv:200510626 2020.
7. Zhao et al. Comput Med Imaging Graph 2020;80.
8. Steeden et al. Journal of Cardiovascular Magnetic Resonance 2020;22(1).
9. Lim et al. CVPR 2017. p136-44.
10. Ledig et al. CVPR 2017. p4681-90.
11. Russakovsky et al. IJCV 2015;115(3).
Figures
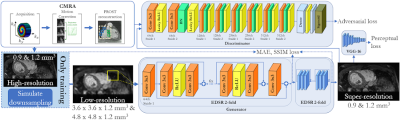
Fig. 1: Proposed generative adversarial super-resolution (SR) framework with
cascaded Enhanced Deep Residual Network for SR (EDSR) generator, trainable
discriminator and perceptual loss network. Non-rigid motion-compensated CMRA
data is acquired to form the low-resolution (1.2x3.6x3.6mm3 or 1.2x4.8x4.8mm3;
superior-inferior x left-right x anterior-posterior) input image/patch which is reconstructed to the
high-resolution output (0.9mm3 or 1.2mm3).
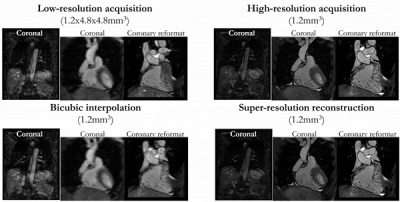
Fig. 2 [animated]: Prospective SR
reconstruction: Coronal and coronary reformat of low-resolution acquisition
(1.2x4.8x4.8mm3) acquired in ~50s, high-resolution acquisition
(1.2mm3) acquired in ~7min, bicubic interpolation (1.2mm3),
and proposed super-resolution reconstruction (1.2mm3) in a patient
with suspected CAD for a prospective acquired low-resolution scan of ~50s scan
time (prospective cohort).
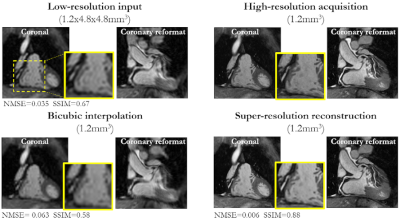
Fig. 3: Retrospective SR reconstruction: Coronal and coronary reformat
of low-resolution input (1.2x4.8x4.8mm3), high-resolution acquisition
(1.2mm3), bicubic interpolation (1.2mm3), and
super-resolution reconstruction (1.2mm3) in a patient with suspected
CAD for a retrospective low-resolution downsampling (retrospective cohort). Zoomed
in image of coronary is shown. Normalized mean squared error (NMSE) and
structural similarity index (SSIM) state differences between images and
high-resolution CMRA.
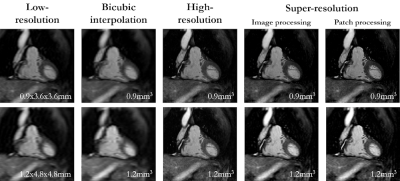
Fig. 4: Patch vs. image-based SR reconstruction: Coronal low-resolution
input, high-resolution acquisition, bicubic interpolation, and super-resolution
reconstruction in a patient with suspected CAD in the retrospective cohort. The
patient was scanned twice with a resolution of 0.9mm3 and 1.2mm3.
Image resolutions are stated below. Patch- and image-wise super-resolution is
compared. Network generalizes for different resolutions and image-/patch-level
processing.
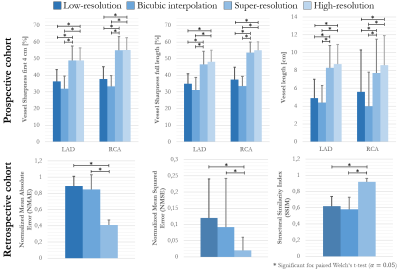
Fig. 5: Quantitative
evaluation of vessel sharpness and vessel length in LAD and RCA of
low-resolution acquisition (1.2x4.8x4.8mm3,
~50s scan), bicubic interpolation (1.2mm3), super-resolution (1.2mm3)
and high-resolution acquisition (1.2mm3, ~7min scan) in 5 patients
of prospective cohort.
NMAE, NMSE and SSIM between low-resolution downsampling (0.9x3.6x3.6mm3, 1.2x4.8x4.8mm3),
bicubic interpolation and super-resolution to high-resolution target (0.9mm3,
1.2mm3) in 5 retrospective patients.