0448
Development, Validation, and Application of an Automated Deep Learning Workflow for Strain Analysis based on cine-MRI1Health Sciences & Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Radiology, Athinoula A. Martinos Center for Biomedical Imaging, MGH, HMS, Charlestown, MA, United States, 3Radiology, University Medical Center Groningen, Groningen, Netherlands, 4Cardiovascular Research Center, MGH, HMS, Charlestown, MA, United States, 5Cardiology, Massachusetts General Hospital, Boston, MA, United States, 6Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States
Synopsis
Myocardial strain analysis from cinematic magnetic resonance imaging (cine-MRI) data is a promising technique for earlier detection of subclinical dysfunction prior to reduction in left-ventricular ejection fraction (LVEF), but sources of discrepancies including user-related variations have limited its wide clinical adoption. Using healthy and cardiovascular disease (CVD) subjects (n=150) we developed a fast, user-independent deep-learning-based workflow for strain analysis from cine-MRI data. Relative to a reference tagging-MRI method, there was no significant difference in end-systolic global strain based on subject-paired cine-MRI data from 15 heathy subjects. Applications in CVD subjects without reduced LVEF showed both global and asymmetric strain abnormalities.
INTRODUCTION
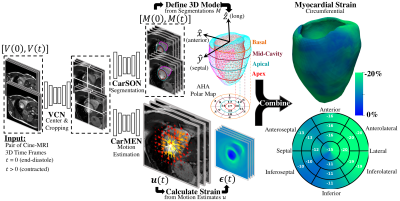
There is an urgent need for early recognition and treatment of subclinical cardiac dysfunction which precedes changes in ejection fraction (EF) and the development of heart failure and other cardiovascular diseases (CVD). Myocardial strain analysis from cinematic magnetic resonance imaging (cine-MRI) data could provide a more thorough characterization of left-ventricular function than EF1, but sources of variation including segmentation and motion estimation have limited its wide clinical use2. Although promising methods using convolutional neural networks (CNN) have been proposed to address these challenges3–8, the accuracy of CNN-based analysis relative to reference standard tagging-MRI is not yet clear. We propose a CNN-based workflow (Fig. 1), termed DeepStrain, consisting of a ventricular centering network (VCN), a cardiac segmentation network (CarSON), and a cardiac motion estimation network (CarMEN). The synergistic combination of these networks enabled us to fully-automatically derive left-ventricular global circumferential (GCS) and radial (GRS) strain from cine-MRI data, which we validated against a tagging-MRI reference method9. We also performed global and regional analyses in cardiovascular disease (CVD) subjects with preserved EF.METHODS
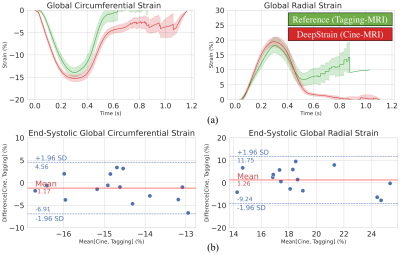
We designed and trained all networks using healthy and CVD subjects3. VCN was trained (n=100) to center and crop input data. CarSON was trained (n=100) to generate labels of the left- and right-ventricular cavities and left-ventricular myocardium, which we used to construct a coordinate system for automated analyses. CarMEN was trained (n=150) to generate estimates from a pair of stacked cine-MRI frames which we used to calculate strain. We compared CarSON-based and manual segmentations from 50 healthy and CVD subjects using the Dice Similarity Coefficient (DSC), and DeepStrain was validated by comparing tagging- and cine-based end-systolic global strain from 15 healthy subjects10 with paired data using Bland-Altman plots. Finally, we applied DeepStrain to 100 training subjects evenly divided into five groups: healthy and patients with hypertrophic cardiomyopathy (HCM), abnormal right ventricle (ARV), myocardial infarction (MI), and dilated cardiomyopathy (DCM)3.RESULTS
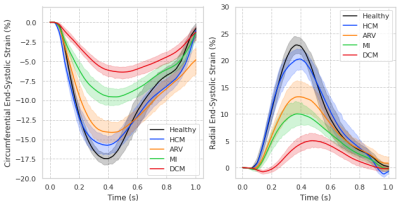
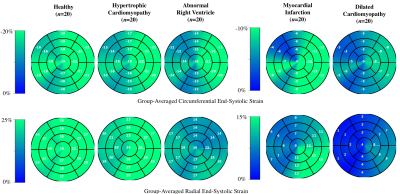
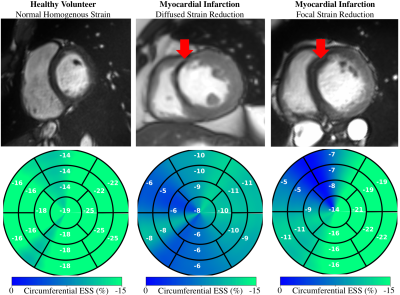
Centering, segmentation, and motion estimation for an entire cardiac cycle (~25 frames) was accomplished in <13s on 12GB GPU and <2.2 min on a 32 GB RAM CPU. VCN located the left-ventricular center with a median error of 1.3 mm. DSC values were 0.97±0.02 and 0.94±0.05 for the left- and right-ventricular cavities, and 0.90±0.03 for the left-ventricular mass. Tagging- vs. cine-based measures were comparable throughout the cardiac cycle (Fig. 2a), and biases in GCS (-14.2±2.2% vs. -15.3±1.5%; bias=-1.17±2.93%) and GRS (18.4±5.1% vs. 19.7±3.4%; bias=+1.26 ± 5.37%) were small and not significantly (p>0.5) different from zero (Fig. 2b).Global strain was reduced in patient groups (Fig. 3), i.e., relative to healthy end-systolic strain (-17.8±1.61%GCS, 24.5±2.82%GRS), there was a progressive decline beginning with HCM (-15.8±2.73%, 20.9±4.68%), followed by ARV (-14.9±2.75%, 15.4±5.50%), MI (-9.8±2.43%, 11.7±4.80%), and DCM (-6.4±1.83%, 5.3±3.24%). Group-averaged regional analyses showed a similar trend (Fig. 4). Healthy values were mostly homogenous across all segments of GCS and GRS polar maps. In HCM, and to a greater extend ARV, GCS was reduced in the septal polar map segments, and across all segments for GRS. In MI and DCM there was considerably greater strain decline, predominantly in the anteroseptal wall in MI and across all segments in DCM. Healthy strain values were also mostly homogenous at an individual level, whereas in two MI patients with septal infarcts (Fig. 5, red arrows) strain reduction was global in one patient, and focally localized to the anteroseptal segments in the other.
DISCUSSION
Quantification of abnormal deformation requires accurate definition of normal ranges either from tagging- or cine-MRI data, but previously reported ranges vary largely between modalities and techniques11. DeepStrain generated end-systolic global strain values on validation data with small standard deviations (±1.5%GCS, ±3.4%GRS), comparable to reference tagging-based values, and in agreement with previous tagging-based studies in healthy participants for GCS (-16.6%, n=12912; -16.7%, n=38613) and GRS (26.5%12; 23.8%13). Further, our analysis was done two orders of magnitude faster than the reference method (1-2h), which makes it an applicable technique for larger datasets, or for daily clinical practice without interfering with the clinical workflow.Application to patient populations showed DeepStrain-based strain analyses could potentially overcome EF limitations14, as these analyses suggested the presence of subclinical left-ventricular systolic dysfunction in HCM and ARV (Fig. 3), i.e., abnormal deformation despite preserved EF15. Regional analyses also suggested the strain abnormalities in HCM were asymmetric (i.e., septal) instead of global (Fig. 4), which agrees with previous cine-MRI-based strain studies in HCM patients16. Asymmetric involvement with HCM is the most common form of the disease, and these results could potentially be indicative of early septal fibrosis1. At an individual level we showed polar map segments with GCS reduction matched infarcted regions in cine-MRI images of MI patients, and that strain reduction can be diffused or focal (Fig. 5). Importantly, from a diagnostic perspective, the global and regional abnormalities demonstrated in this study could potentially be used to discriminate dysfunctional from functional myocardium18, or as interpretable imaging phenotypes for CNN-based classification algorithms19,20.
CONCLUSION
We developed and validated a fast, operator-independent CNN-based workflow for strain analysis to quantitatively characterize cardiac mechanics based on ubiquitously acquired cine-MRI data. These technical and practical attributes position DeepStrain as a promising candidate for wide clinical use.Acknowledgements
We acknowledge the support of NVIDIA Corporation with the donation of the Titan X Pascal GPU used for this research.References
1. Claus, P., Omar, A. M. S., Pedrizzetti, G., Sengupta, P. P. & Nagel, E. Tissue Tracking Technology for Assessing Cardiac Mechanics. JACC: Cardiovascular Imaging 8, 1444–1460 (2015).
2. Amzulescu, M. S. et al. Head-to-Head Comparison of Global and Regional Two-Dimensional Speckle Tracking Strain Versus Cardiac Magnetic Resonance Tagging in a Multicenter Validation Study. Circ Cardiovasc Imaging 10, (2017).
3. Bernard, O. et al. Deep Learning Techniques for Automatic MRI Cardiac Multi-Structures Segmentation and Diagnosis: Is the Problem Solved? IEEE Trans. Med. Imaging 37, 2514–2525 (2018).
4. Puyol-Anton, E. et al. Fully automated myocardial strain estimation from cine MRI using convolutional neural networks. in 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018) 1139–1143 (IEEE, 2018). doi:10.1109/ISBI.2018.8363772.
5. Qin, C. et al. Joint Learning of Motion Estimation and Segmentation for Cardiac MR Image Sequences. arXiv:1806.04066 [cs] (2018).
6. Qiao, M. et al. Temporally coherent cardiac motion tracking from cine MRI: Traditional registration method and modern CNN method. Med. Phys. 47, 4189–4198 (2020).
7. Yu, H. et al. FOAL: Fast Online Adaptive Learning for Cardiac Motion Estimation. in 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) 4312–4322 (IEEE, 2020). doi:10.1109/CVPR42600.2020.00437.
8. Chen, P. et al. Anatomy-Aware Cardiac Motion Estimation. arXiv:2008.07579 [cs, eess] (2020).
9. Tautz, L., Hennemuth, A. & Peitgen, H.-O. Motion Analysis with Quadrature Filter Based Registration of Tagged MRI Sequences. in Statistical Atlases and Computational Models of the Heart. Imaging and Modelling Challenges (eds. Camara, O. et al.) vol. 7085 78–87 (Springer Berlin Heidelberg, 2012).
10. Tobon-Gomez, C. et al. Benchmarking framework for myocardial tracking and deformation algorithms: An open access database. Medical Image Analysis 17, 632–648 (2013).
11. Venkatesh, B. A. et al. Regional myocardial functional patterns: Quantitative tagged magnetic resonance imaging in an adult population free of cardiovascular risk factors: The multi-ethnic study of atherosclerosis (MESA): Reference Values of Strain From Tagged MRI. J. Magn. Reson. Imaging 42, 153–159 (2015).
12. Ferdian, E. et al. Fully Automated Myocardial Strain Estimation from Cardiovascular MRI–tagged Images Using a Deep Learning Framework in the UK Biobank. Radiology: Cardiothoracic Imaging 2, e190032 (2020).
13. Konstam, M. A. & Abboud, F. M. Ejection Fraction: Misunderstood and Over-rated (Changing the Paradigm in Categorizing Heart Failure). Circulation 135, 717–719 (2017).
14. Khan, J. N. et al. Subclinical diastolic dysfunction in young adults with Type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. European Heart Journal - Cardiovascular Imaging 15, 1263–1269 (2014).
15. Xu, H. et al. Early marker of regional left ventricular deformation in patients with hypertrophic cardiomyopathy evaluated by MRI tissue tracking: The effects of myocardial hypertrophy and fibrosis: MR Tissue Tracking of HCM. J. Magn. Reson. Imaging 46, 1368–1376 (2017).
16. Chun, E. J. et al. Hypertrophic Cardiomyopathy: Assessment with MR Imaging and Multidetector CT. RadioGraphics 30, 1309–1328 (2010).
17. Götte, M. J. W. et al. Quantification of regional contractile function after infarction: strain analysis superior to wall thickening analysis in discriminating infarct from remote myocardium. Journal of the American College of Cardiology 37, 808–817 (2001).
18. Zhang, N. et al. Deep Learning for Diagnosis of Chronic Myocardial Infarction on Nonenhanced Cardiac Cine MRI. Radiology 291, 606–617 (2019).
19. Zheng, Q., Delingette, H. & Ayache, N. Explainable cardiac pathology classification on cine MRI with motion characterization by semi-supervised learning of apparent flow. arXiv:1811.03433 [cs, stat] (2019).
Figures