0440
k-Space Weighted Image Average (KWIA) for ASL-based Dynamic MRA and Perfusion Imaging1Laboratory of Functional MRI Technology (LOFT), Mark & Mary Stevens Neuroimaging and Informatics Institute, University of Southern California, Los Angeles, CA, United States
Synopsis
The intrinsically low SNR of ASL techniques is a main limitation that hinders their clinical translations. This work presented a novel denoising algorithm for dynamic MRI termed KWIA and evaluated its use in multi-delay ASL and ASL-based 4D dMRA. KWIA improves SNR without compromising spatial and temporal resolution by progressively increasing the neighboring time frames for view-shared averaging for more distal k-space regions. Experimental results showed that KWIA can provide significant SNR improvement that enables better visualization and quantification for both multi-delay ASL and ASL-based 4D dMRA as well as other dynamic MRI techniques.
Introduction
Arterial spin labeling (ASL) offers noninvasive measurement of cerebral blood flow (CBF) and can be applied for non-contrast time-resolved 4D dynamic MRA (dMRA). In particular, multi-delay ASL enables the visualization of flow dynamics and quantification of multiple hemodynamic parameters1,2. However, the intrinsically low signal-to-noise ratio (SNR) hampers the clinical utility of ASL techniques. The purpose of this study is to present a novel denoising algorithm termed k-space weighted image average (KWIA)3 originally proposed for low dose CT perfusion to improve the SNR of dynamic MRI without compromising the spatial and temporal resolution or quantification accuracy. In this study, we first presented the theoretical framework of KWIA and evaluated its performance in terms of SNR, temporal blurring, and quantification on in vivo data.Theory
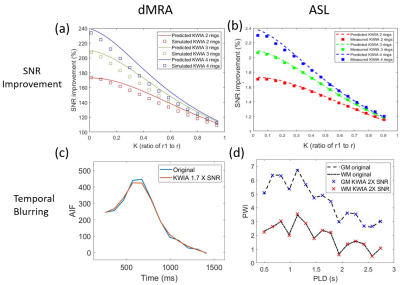
KWIA is a retrospective denoising method that divides the k-space into multiple rings, as shown in Figure 1(a). A symmetric moving averaging window with a window size determined by N, the number of rings, slides from the beginning to the end of the time series. When the KWIA window is applied to a selected time frame, the central ring of this k-space remains intact to preserve the image contrast and temporal resolution, while outer rings are progressively averaged between neighboring time frames to increase SNR. As shown in Figure 1(b), the expected SNR improvement by KWIA increases with a greater N, and a smaller K, the ratio of the central ring to the full radius, under the assumption that the noise in k-space is uniformly distributed additive Gaussian noise with zero-mean. Unlike project view-sharing techniques such as k-space weighted image contrast (KWIC)4, KWIA can be applied on data acquired with any k-space trajectory or order. KWIA can also be directly applied to raw k-space data or that converted from MR images by FFT. The KWIA-filtered k-space data can then be converted to denoised MRI using inverse FFT. Since the image contrast is primarily determined by central k-space, KWIA improves SNR while preserving the temporal and spatial resolution of dynamic MRI.Methods
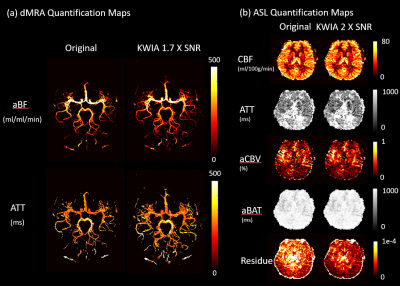
A Shepp-Logan digital phantom with simulated noise was created to evaluate the performance of KWIA under various simulated MRI conditions listed in Figure 1(c). The ASL data was acquired on a Siemens Prisma 3T scanner using a 15-delay pCASL protocol with 3D GRASE readout (PLD=500:160:2740 ms, voxel size=3×3×4 mm, matrix=64×64×30, single repetition, total 10min). The dMRA data was acquired on a Siemens Tim Trio 3T scanner (bSSFP-based multi-phase 3D Cartesian acquisition, 22 time frames, PLD=225:105:2445 ms, voxel size=1×1×1.5 mm, 75% partial FT along phase directions; in-plane GRAPPA with R=2, matrix=220×176×32, total 6min). KWIA was applied with the following (N, K) configurations: (2, 0.58), (3, 0.4), and (3, 0.18) to gain 1.4, 1.7, and 2 folds SNR increase, respectively. The temporal blurring effect was evaluated using arterial input function (AIF) from the middle cerebral artery (MCA) for dMRA and subtracted perfusion signals from gray matter (GM) and white matter (WM) for ASL. For quantification, arterial blood flow (aBF) and arterial transit time (ATT) were calculated using Block-circulant singular value decomposition (cSVD)5 for dMRA (with 15% threshold). Moreover, CBF, ATT, arterial cerebral blood volume (aCBV), arterial bolus arrival time (aBAT), and residual maps were derived using two-compartment model fitting6 for 15-delay pCASL.Results and discussion
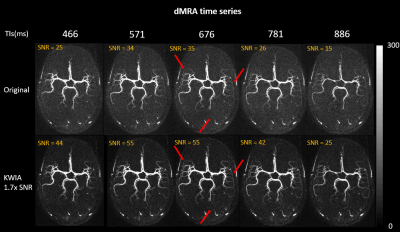
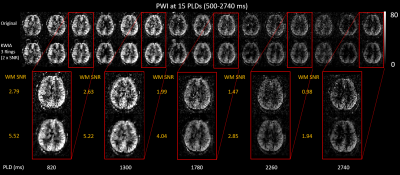
As shown in Figure 1(c), the SNR improvements using KWIA match the predicted ratios under most simulated MRI conditions, except for k-space filtering and zero-filling, where a small drop (~10%) was observed. Figure 2 shows five consecutive time frames of dMRA MIP images with and without 1.7-fold-SNR KWIA. KWIA revealed distal vasculatures that were overwhelmed by noise in original dMRA. This reappearance of distal vasculatures due to improved SNR can be differentiated from the temporal blurring of neighboring frames because no such structures can be visually captured within the KWIA window. Figure 3 shows perfusion images of 15-delay pCASL with and without 2-fold-SNR KWIA. KWIA significantly improved image quality and visualization of dynamic perfusion signals without introducing notable spatial and temporal smoothing. Figure 4 (a) and (b) demonstrate the excellent consistency between the measured SNR improvement and the predicted ratio for dMRA and ASL data, respectively. In Figure 4 (c) and (d), despite a 5% signal drop at the peak of AIF of dMRA, the AIF of dMRA and GM/WM signal of ASL was not severely affected by KWIA with NRMSE of 0.024 and 0.008/0.023, respectively, suggesting negligible temporal blurring was introduced. Figure 5 (a) and (b) display the quantitative parametric maps of dMRA and ASL, respectively. KWIA did not adversely affect the quantification results. Instead, benefiting from the improved SNR, KWIA recovered the missing distal vasculatures in original aBF and ATT maps for dMRA and suppressed the residue for ASL.Conclusion
A novel denoising algorithm for dynamic MRI termed KWIA was presented to improve SNR of ASL-based dMRA and perfusion without compromising the spatial and temporal resolution or quantification accuracy. KWIA is applicable to other dynamic MRI techniques such as dynamic susceptibility contrast (DSC) and dynamic contrast enhanced (DCE) MRI.Acknowledgements
No acknowledgement found.References
1. Wang D, Alger J, Qiao J, et al. Multi-delay multi-parametric arterial spin-labeled perfusion MRI in acute ischemic stroke — Comparison with dynamic susceptibility contrast enhanced perfusion imaging. NeuroImage: Clinical. 2013;3:1‐7.
2. Yan L, Wang S, Zhuo Y, et al. Unenhanced dynamic MR angiography: high spatial and temporal resolution by using true FISP-based spin tagging with alternating radiofrequency. Radiology. 2010;256(1):270‐279.
3. Zhao C, Martin T, Shao X, et al. Low Dose CT Perfusion with K-Space Weighted Image Average (KWIA). IEEE Transaction on Medical Imaging. 2020;39(12):3879-3890.
4. Song H, Dougherty L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2004;52(4):815-824.
5. Wu O, Ostergaard L, Weisskoff R, et al. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2003;50(1):164-174.
6. Chappel M, MacIntosh, B, Okell, T, et al. Introduction to perfusion quantification using arterial spin labelling. Oxford University Press. 2018;
Figures