0436
Abnormal Oxidative Metabolism in the Gray Matter of Cuprizone Mouse Model: An in-vivo NIRS-MRI Study1Department of Radiology, University of Calgary, Calgary, AB, Canada, 2Biomedical Engineering Graduate Program, University of Calgary, Calgary, AB, Canada, 3Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 4Experimental Imaging Centre, University of Calgary, Calgary, AB, Canada, 5Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Synopsis
Non-invasive quantitative imaging of cerebral oxygen consumption is crucial to understand the involvement of oxidative metabolism in neurological diseases. We are applying a novel multimodal technique combining near-infrared spectroscopy and high-field MRI to study the mitochondrial status as well as oxygen delivery and consumption in the cortex of the cuprizone mouse model. In this study, multiple physiological parameters controlling oxidative metabolism were investigated in the cuprizone mice exhibiting demyelination. A mitochondrial impairment and a reduced oxygen consumption rate were found in the gray matter of cuprizone mice, emphasizing the association between abnormal oxidative metabolism and the observed demyelination.
INTRODUCTION
Disruptions in oxidative metabolism may occur in neurological diseases, such as Multiple Sclerosis (MS)1,2, Alzheimer's Disease3, Parkinson’s disease4. Non-invasive examination of oxidative metabolism and the multiple physiological parameters involved in this process, such as oxygenation levels, dynamics of the blood flow, and mitochondrial status, is crucial to further understand the role of these parameters in neurological diseases. We are implementing a novel multimodal imaging technique combining Near-infrared Spectroscopy (NIRS) and MRI to study simultaneously, and non-invasively, physiological alterations in the brain of mouse models. Using this technique, we are monitoring oxygenation (StO2) and mitochondrial capacity focusing on the key enzyme Cytochrome C Oxidase (CCO), while providing information on the process of blood delivery (CBF) and O2 consumption (CMRO2) throughout the brain of the cuprizone mouse model. This is a well-known model for the study of demyelination and spontaneous remyelination, which is also useful to study human MS5.METHODS
Twenty C57BL/6J male mice (8-week-old) were separated into Control (n = 9) and Cuprizone (n = 11) groups. Control mice received normal mouse chow, while cuprizone mice received a diet of ground chow mixed with cuprizone6. After 5 weeks, cuprizone diet was terminated, and the Cuprizone group was given normal diet for 4 additional weeks, for recovery. NIRS-MRI imaging were performed for both groups prior to the cuprizone diet initiation (Week 0), at the end of the cuprizone exposure (Week 5) and after cuprizone cessation (Week 9). Statistical analysis was performed comparing CPZ and CTRL groups over 3 different time points using mixed factorial ANOVA with Bonferroni post-hoc test. During imaging, the mice were spontaneously ventilated with a gas mixture of 70% N2 and 30% O2 in addition to 2% isoflurane. A 9.4T MRI with a 35mm volume coil was used to quantify Magnetization Transfer Ratio (MTR) as a marker for de/remyelination. A single axial slice was acquired using a spin-echo sequence with the following parameters: TR/TE=2500/15 ms, FOV=12.8x12.8 mm, voxel resolution=100x100x1500 µm3. 40 MT pulses of Gauss shape were applied. The MT pulse B1 strength was 10.0 µT with 15 ms duration, 1ms interpulse delay, 182.7 Hz bandwidth, and an offset frequency of 1500 Hz. An identical reference magnitude image, M0, was collected with no MT pulse. In total, two images (with and without MT pulse) were collected over a period of 12 min, and MTR maps were generated. ROI were selected within the corpus callosum and cerebral cortex. For perfusion measurement, the same slice was acquired using a CASL-HASTE sequence with the following parameters: TR/TE=3000/13.5 ms, FOV=25.6×25.6 mm, matrix size=128×128 pixels, slice thickness=1.5 mm, 16 averages. Four perfusion images were collected per measurement: 2 control images and 2 tagged images, to correct for magnetization transfer. Following these images, a T1 map was obtained in the same location using a RARE-VTR sequence where effective TE=20 ms, TR=100, 500, 1000, 3000, and 7500 ms. Together, the four perfusion images and the T1 map were collected over a period of 14 min. CBF was calculated on a voxel-by-voxel basis7. In addition, a two-dimensional T2-weighted RARE sequence was acquired with TR/TE=3000/32 ms, FOV=25.6x25.6 mm, matrix size=256x256, voxel resolution=100x100 µm2, slice thickness=0.5 mm, 10 averages, and acquisition time=16 min. We measured the concentration of hemoglobin and StO2 in mouse cortex, in addition to the concentration and redox state of CCO using a custom-built broadband NIRS device and in-house developed processing algorithms. CMRO2 was calculated based on the modified Fick principle8.RESULTS
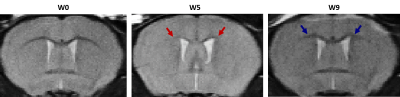
Cuprizone mice showed reduced OEF (31.8%, p≤0.05) and CMRO2 (47.2%, p≤0.001) that were resolved after cessation of cuprizone exposure, in addition to a decrease in hemoglobin concentration (28.42%, p≤0.05), an increase in tissue oxygenation (5.7%, p≤0.05) but no change in CBF. The oxidized state of CCO increased (36.9%, p≤0.01) significantly in cuprizone mice while the reduced state decreased (34.4%, p≤0.05). The total amount of the enzyme was not affected by the cuprizone diet, however, it decreased at week 9 both in control (23.1%, p≤0.01) and cuprizone (28.8%, p≤0.01) groups. A reduced value of MTR at week 5 was observed in the cuprizone group both in cerebral cortex (3.2%, p≤0.05) and corpus callosum (5.1%, p≤0.001). T2-weighted images of cuprizone mice showed a strong gray-white matter contrast which decreased at week 5 and increased back after recovery.DISCUSSION
Higher levels of oxidized CCO were found in cuprizone mice, which could be an adaptation in response to an impairment in the electron transport chain. The abnormal redox state of CCO led to lower O2 extraction fraction, lower O2 consumption rate by the tissue, and thus a higher O2 availability (StO2) in cortical microvessels. These metabolic changes were associated with the impaired myelination occurring in the gray matter (GM) as well as the white matter of the cuprizone mouse model.CONCLUSION
We were able to detect significant metabolic alterations in the GM of cuprizone mice using novel NIRS-MRI multimodality system. Demyelination and possible mitochondrial involvement were supported by reduced MTR, increased redox state of CCO, and reduced CMRO2. The novel multimodal imaging technique applied here shows promise for noninvasively assessing parameters associated with oxidative metabolism in both mouse models of neurological disease and for translation to study oxidative metabolism in human brain.Acknowledgements
This work was supported by an NIH R21 grant, Canada Foundation for Innovation (CFI), Natural Sciences and Engineering Research Council (NSERC), Discovery grant, and the Biomedical Engineering Graduate Program (BMEG) at U of C.References
1. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787-795.
2. Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain: a journal of neurology. Jul 2008;131(Pt 7):1722-1735.
3. Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2010;1802(1):2-10.
4. Cai H, Cong W-n, Ji S, Rothman S, Maudsley S, Martin B. Metabolic dysfunction in Alzheimer's disease and related neurodegenerative disorders. Current Alzheimer research. 2012;9(1):5-17.
5. Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P. Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neuroscience & Biobehavioral Reviews. 2014;47:485-505.
6. Ghaiad HR, Nooh MM, El-Sawalhi MM, Shaheen AA. Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: Biochemical and histological study. Molecular neurobiology. 2017;54(5):3219-3229.
7. Buxton RB. Quantifying CBF with arterial spin labeling. Journal of magnetic resonance imaging : JMRI. Dec 2005;22(6):723-726.
8. Tichauer KM, Hadway JA, Lee T-Y, Lawrence KS. Measurement of Cerebral Oxidative Metabolism with Near-Infrared Spectroscopy: A Validation Study. Journal of Cerebral Blood Flow & Metabolism. 2006;26(5):722-730.
Figures