0434
Quantitative neuroimaging biomarkers using 3D UTE MRI and ferumoxytol1Imaginostics, Inc., Cambridge, MA, United States, 2Center for Translational Neuroimaging, Northeastern University, Boston, MA, United States, 3Medical Physics, University of Wisconsin–School of Medicine and Public Health, Madison, WI, United States, 4Martinos Center, Massachusetts General Hospital and Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States, 5Massachusetts General Hospital, Boston, MA, United States, 6University of California, San Francisco, San Francisco, CA, United States
Synopsis
New quantitative vascular neuroimaging biomarkers are made possible by the combination of optimized 3D UTE MRI and ferumoxytol for contrast enhancement. Here, we have shown that this method, QUTE-CE MRI, can be used to image both the vascular and perivascular space. The small vessel density biomarker demonstrated sensitivity to hyper-to-normal microvascularization from 8-24 months of age in an ApoE4 genetic knock-in rat model. Disruption in the blood brain barrier was detected in individual animals and groups after even a single mild hit to the head. Finally, feasibility of QUTE-CE MRI is demonstrated on clinical scanners for human imaging.
INTRO
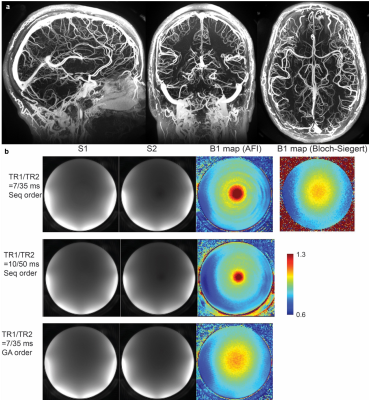
This research aims to develop a recently established MRI-based vascular imaging methodology: quantitative ultra-short time-to-echo contrast-enhanced magnetic resonance imaging (QUTE-CE MRI)1-5. We hypothesized that the biomarkers would reveal site-specific structural and functional vascular abnormalities in an ApoE4 genetically modified rat model, blood-brain barrier (BBB) leakage in a repetitive-mild traumatic brain injury (rmTBI) model. Finally, we sought to demonstrate clinical feasibility by obtaining high resolution positive-contrast vascular angiograms and accurate B1+ mapping with pulse sequences developed for human use.METHODS
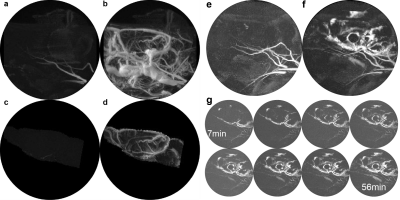
Small animal neuroimaging: Images were obtained using a Bruker Biospec 7.0 T/20 cm USR horizontal magnet (Bruker, Billerica, MA, USA) equipped with a 20-G/cm magnetic field gradient insert (ID=12 cm, Bruker). On the day of imaging, rats were anesthetized with 2-3% isoflurane, fixed with a tail-vein catheter, and secured into a custom-built restraining system.Visualizing dynamic mixing in the perivascular space: To access intrathecal injection, the scalp was incised and a burr hole made in the skull for implantation of sterile PE10 tubing (Braintree Scientific) aimed at the right lateral cerebroventricle using the stereotaxic coordinates: 1.0 mm posterior to the bregma, 2.0 mm lateral to the midline, and 4.0 mm in depth from dura. The tubing, ca 60 cm in length and prefilled with Ferumoxytol, was fixed in place with cyanoacrylic cement and connected to a 0.3 mL syringe needle filled with the contrast agent. 3D UTE imaging began immediately after ferumoxytol injection (20ul at 10 mg/ml Fe).
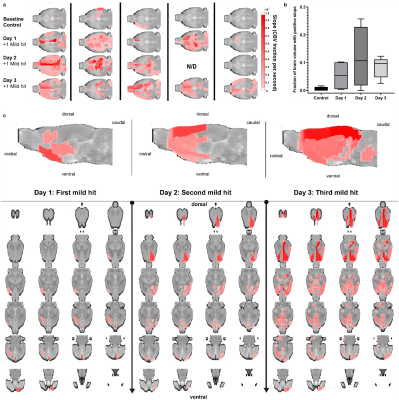
Detecting rmTBI: A closed-head momentum exchange model was used to produce mild head injuries in male Sprague Dawley (SD) rats as compared to non-injured controls. N=5 SD rats were scanned at 24hr increments before (baseline control) and immediately following 1 mild hit per day. Concussion were generated with a pneumatic pressure drive, 50 g compactor described by Viano and colleagues6 and refined by Mychasiuk et al7 to reliably produce the 7.4 m/s impact velocities described for mild rat head injury.8 The impact piston was directed to the top of the skull, midline, in the approximate area of Bregma. All control and TBI rats were anesthetized with 2% isoflurane. Rats were awake and ambulatory within 5-7 min after anesthesia and concussion.
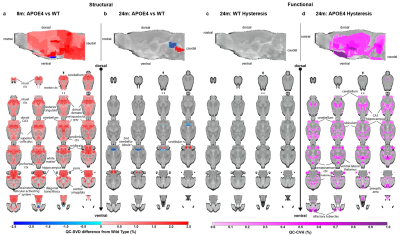
Vascular abnormality in APOE4 genotype: This study was performed from a cross-sectional sample of ApoE4 genetic knock-in rats and their WT age-matched littermate controls. Images were obtained at 8 and 24 months of age. At 8 months, two groups (n=6 WT and n=5 APOE4) were imaged for structural biomarkers only, and at 24 months, two groups (n=5 WT, n=6 APOE4) were imaged for both structural and functional biomarkers. During the scanning session, rats were given an IV bolus of 14 mg/kg Fe. At 8 months, three averages pre- and post-contrast were obtained using structural biomarkers with high signal-to-noise ratios (SNR). At 24 months, imaging was performed without averaging while cycling on and off a 5% CO2 hypercapnic challenge after the first post-contrast image.
RESULTS
Dynamic Mixing in the Perivascular Space: The QUTE-CE MRI method with ferumoxytol produces unique angiographic images with a quantitative signal that are independent of flow (Fig 1a-d). Ferumoxytol injected intrathecally mixes relatively slowly throughout the cerebral spinal fluid and is visualized during an hour-long period (Fig. 1e-g).rmTBI: A progressive increase in BBB leakage was found after rmTBI (Fig. 2), and the effect of rmTBI differentially impacted each cerebral hemisphere. Notably, the somatosensory cortex, secondary and primary motor cortex were significantly impacted on the right cerebral hemisphere after rmTBI, whereas, the effect on the thalamic nuclei was consistent across both hemispheres. Additionally, the CBV vs. time course could be used to detect BBB leakage in individuals.
Vascular abnormality in APOE4 genotype: After correction for multiple comparisons, APOE4 rats present with significant hyper-microvascularity at 8 months of age, which is reduced at 24 months. At 24 months, APOE4 rats also display hysteretic use of the vascular reserve.
DISCUSSION
Ferumoxytol, an FDA-approved iron-oxide nanoparticle formulation for treating iron deficiency anemia, is particularly synergistic as a contrast agent for this method since it is both intravascular and has a long blood half-life (15h vs. 15-30min for gadolinium-based contrast agents). Consequently, the technique produces unique angiograms that are renal-safe.Results demonstrate that QUTE-CE MRI can be used to visualize mixing in the perivascular space/glymphatic system over a 1-hour period, detect BBB leakage in individual animals, or in groups, using a rmTBI model. Results from ApoE4 animals suggest that early hyper-vascularization may be a mechanism for metabolic resilience, and that a combination of structural and functional vascular imaging biomarkers may aid assessment of neurovascular dysfunction in ADRDs.
CONCLUSION
QUTE-CE MRI offers new possibilities with vascular imaging biomarkers for precision medicine.Acknowledgements
No acknowledgement found.References
1. Gharagouzloo, C.A., McMahon, P.N. & Sridhar, S. Quantitative contrast-enhanced MRI with superparamagnetic nanoparticles using ultrashort time-to-echo pulse sequences. Magn Reson Med 74, 431-441 (2015).
2. Gharagouzloo, C.A., et al. Quantitative vascular neuroimaging of the rat brain using superparamagnetic nanoparticles: New insights on vascular organization and brain function. Neuroimage 163, 24-33 (2017).
3. van de Ven, A.L., et al. Nanoformulation of Olaparib Amplifies PARP Inhibition and Sensitizes PTEN/TP53-Deficient Prostate Cancer to Radiation. Mol Cancer Ther 16, 1279-1289 (2017).
4. Gharagouzloo, C.A., et al. Dataset on a 173 region awake resting state quantitative cerebral blood volume rat brain atlas and regional changes to cerebral blood volume under isoflurane anesthetization and CO. Data Brief 17, 393-396 (2018).
5. Qiao, J., Lawson, C.M., Rentrup, K.F.G., Kulkarni, P. & Ferris, C.F. Evaluating blood-brain barrier permeability in a rat model of type 2 diabetes. J Transl Med 18, 256 (2020).
6. Viano, D.C., Hamberger, A., Bolouri, H. & Saljo, A. Concussion in professional football: animal model of brain injury--part 15. Neurosurgery 64, 1162-1173; discussion 1173 (2009).
7. Mychasiuk, R., Hehar, H., Candy, S., Ma, I. & Esser, M.J. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J Neurosci Methods 257, 168-178 (2016).
8. Kulkarni, P., et al. Neuroradiological Changes Following Single or Repetitive Mild TBI. Front Syst Neurosci 13, 34 (2019).
Figures