0433
Sensitive imaging schemes for dynamic glucose enhanced (DGE) MRI to detect glucose uptake and clearance in mouse brain at 3T1Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Research Institute, Baltimore, MD, United States, 3Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4City University of Hong Kong Shenzhen Research Institute, Shenzhen, China
Synopsis
We developed a high-sensitivity dynamic glucose enhanced (DGE) MRI acquisition and post-processing scheme for sensitive monitoring glucose uptake and clearance in both brain parenchyma and cerebrospinal fluid (CSF) at 3T. By investigating Carr-Purcell-Meiboom-Gill sequence (CPMG), on-resonance variable delay multi-pulse (onVDMP) and on-resonance spin-locking (onSL), a high-sensitivity DGE MRI scheme composed of CPMG method for monitoring parenchyma and onVDMP method for monitoring CSF was proposed. We incorporated the multilinear singular value decomposition (MLSVD) based denoising method in post-processing, which enables the detection of DGE signals from the brain parenchyma and CSF at low concentration of D-glucose (12.5% w/w) injection.
Introduction
Abnormal glucose level is a hallmark of many diseases, such as cancer,1,2 diabetes,3,4 and Alzheimer’s disease (AD).5,6 Chemical exchange saturation transfer (CEST) MRI has shown great potential to detect glucose (named glucoCEST) via the exchange mechanism between hydroxyl proton and bulk water non-invasively.7-11 This enables the monitoring of dynamic glucose enhanced (DGE) signal in the brain after intravenous glucose injection, which helps to reveal glucose-related pathologies. Recently, we proposed a new DGE MRI approach, named on-resonance variable delay multi-pulse (onVDMP),12 to efficiently detect glucose in both brain parenchyma and cerebrospinal fluid (CSF) simultaneously.13-15 Other alternatives, such as Carr-Purcell-Meiboom-Gill sequence (CPMG)16,17 and on-resonance spin-locking (onSL),18-20 have shown promising sensitivity on D-glucose detection. By comparing these three methods, we develop a high-sensitivity DGE MRI acquisition scheme for sensitively monitoring glucose uptake and clearance in both brain parenchyma and CSF at clinical field strength (3T). With incorporating the multilinear singular value decomposition (MLSVD) based denoising method21 in post-processing, the proposed DGE MRI can detect DGE signals from the brain parenchyma and CSF with as low as 12.5% w/w D-glucose injection.Methods
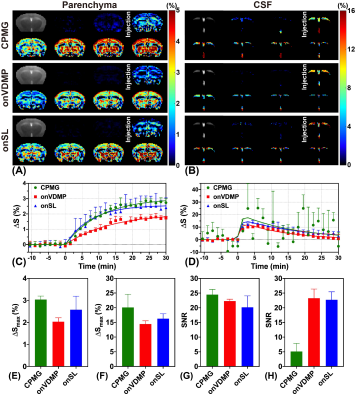
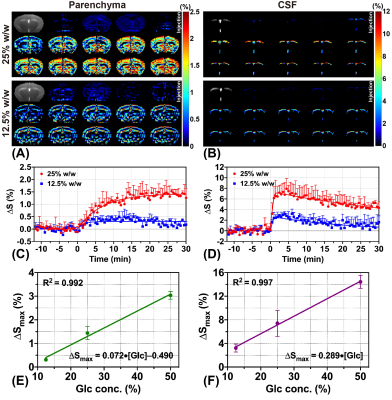
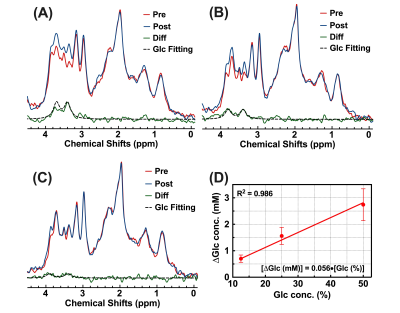
Nine wild type (WT) mice (C57BL/6 female at 10 months old) were used in this study. All MRI experiments were performed on a horizontal bore 3T Bruker Biospec system (Bruker, Ettlingen, Germany). The MRI sequences for animal experiments are shown in Figure 1A. Three different labeling modules (CPMG, onVDMP, and onSL), followed by the rapid acquisition with refocused echoes (RARE) sequence as a readout module. For all three labeling modules, the module lengths (tsat) were adjusted to detect parenchyma and CSF respectively (tsat=60 ms for parenchyma and tsat=900 ms for CSF). Acquisition parameters are as followings: TR/TE= 5 s/5 ms, RARE factor=32, centric encoding, slice thickness=2 mm, a matrix size of 96×96 within a FOV of 20 × 20 mm2 (15 s per image). The total imaging time of each DGE experiment was 42 mins (168 images). During the DGE acquisition, a bolus of 0.15 mL filtered D-glucose (50%/25%/12.5% w/w) was injected into the mouse at 12 min through the tail vein over one minute (speed: 0.15 mL/min). The temporal resolution of the DGE curve for DGE experiments with 50% w/w D-glucose injection was 1 min 30 s (15×6 s) (Figure 1B), while the temporal resolution of DGE curve of low-concentration experiment was 30 s (15×2 s) (Figure 1C). MRS data were obtained from a voxel box (3×3×3 mm3) in thalamus using the stimulated echo acquisition mode (STEAM) pulse sequence with outer volume suppression before and after the DGE imaging.The MLSVD based low-rank reconstruction was adopted for DGE image denoising.21 The percentage change of DGE signal was calculated by$$\triangle S(t_{n})=\frac{S_{base}-S(t_{n})}{S_{base}}$$For the data fitting, an exponential model14 and a sigmoid model22,23 are applied on the DGE curves of parenchyma and CSF respectively, to extract the maximal DGE signal ΔSmax and uptake/clearance rates (μin/μout):$$\triangle S_{fit}^{par}(t_{n})=\triangle S_{max}\cdot[1-e^{-\mu_{in}(t-t_{0})}]$$$$\triangle S_{fit}^{CSF}(t_{n})=\triangle S_{max}\cdot\frac{e^{-\mu_{out}(t-t_{0})}}{1+e^{-\mu_{in}(t-t_{0})}}$$After fitting, the signal-to-noise (SNR) of DGE curves was calculated as24$$SNR=10lg(\frac{\triangle S_{max}^2}{\sigma^{2}})$$$$\sigma^{2}=\frac{1}{N}\sum_{n=1}^N[\triangle S(t_{n})-\triangle S_{fit}(t_{n})]^{2}$$where σ2 represents the noise variance.Results and Discussion
For 50% w/w D-glucose injection, CPMG method sensitively detected the parenchymal DGE signal with the highest ΔSmax of 3.04%±0.16% and SNR of 24.48±1.79. However, the CSF DGE curves acquired by CPMG method showed severe fluctuations (Figure 2D, SNR = 5.13±2.75), indicating that CPMG method was not suitable for CSF DGE study. As for onVDMP and onSL methods, both could detect the CSF DGE signal with high signal changes ΔSmax (Figure 2B, 14.43%±1.11% versus 16.26%±1.67%), while onVDMP method achieved the highest SNR (23.25±3.14). Hence, a high-sensitivity DGE MRI scheme composed of CPMG method for monitoring parenchyma and onVDMP method for monitoring CSF was used in DGE experiments with low-concentration D-glucose injection (Figure 1C). Results showed that the high-sensitivity DGE scheme can achieve a ΔSmax of 1.44%±0.28% for parenchyma and a ΔSmax of 7.43%±2.21% for CSF at 25% w/w D-glucose injection (Figure 3C,D). When the injected D-glucose concentration was reduced to 12.5% w/w, a parenchymal ΔSmax of 0.31%±0.06% was detected by CPMG and a CSF ΔSmax of 3.23%±0.68% was detected by onVDMP (Figure 3C,D). After fitting all the ΔSmax data, D-glucose concentration dependence of ΔSmax was found to be linear in both parenchyma (R2=0.992) and CSF (R2=0.997) (Figure 3E,F). From MRS validation results, we can see a glucose concentration increment of 2.74±0.60 mM was observed for 50% D-glucose injection, while glucose concentration increments were found to be 1.56±0.32 mM and 0.70±0.14 mM for 25% and 12.5% D-glucose injections, respectively (Figure 4). These results echoed our DGE findings.Conclusion
Our study demonstrated that a sensitive DGE MRI scheme can detect glucose uptake and clearance in mouse brain at a low concentration of D-glucose (12.5% w/w) injection. It is composed of CPMG method for monitoring parenchyma and onVDMP method for monitoring CSF. Combining the MLSVD based low-rank reconstruction, the proposed DGE method was able to acquire DGE images with high SNR and sensitivity. This could be a robust scheme for monitoring cerebral glucose in clinical applications.Acknowledgements
Research Grants Council: 11102218; City University of Hong Kong: 7005210, 9680247, 9667198 and 6000660; National Natural Science Foundation of China: 81871409.References
1. Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol 2006;18(6):598-608.
2. Tayek JA. A review of cancer cachexia and abnormal glucose metabolism in humans with cancer. J Am Coll Nutr 1992;11(4):445-456.
3. Vashist SK. Non-invasive glucose monitoring technology in diabetes management: A review. Anal Chim Acta 2012;750:16-27.
4. Geijselaers SL, Sep SJ, Stehouwer CD, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015;3(1):75-89.
5. Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. Eur J Nucl Med Mol I 2005;32(4):486-510.
6. An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O, Chia CW, Egan JM, Ferrucci L, Troncoso J. Evidence for brain glucose dysregulation in Alzheimer's disease. Alzheimers Dement 2018;14(3):318-329.
7. Chan KW, McMahon MT, Kato Y, Liu G, Bulte JW, Bhujwalla ZM, Artemov D, van Zijl PC. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med 2012;68(6):1764-1773.
8. Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Gonçalves M, Parkes HG, Årstad E. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 2013;19(8):1067-1072.
9. Van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 2011;65(4):927-948.
10. Ward K, Aletras A, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000;143(1):79-87.
11. van Zijl PC, Lam WW, Xu J, Knutsson L, Stanisz GJ. Magnetization transfer contrast and chemical exchange saturation transfer MRI. Features and analysis of the field-dependent saturation spectrum. NeuroImage 2018;168:222-241.
12. Xu J, Chan KW, Xu X, Yadav N, Liu G, van Zijl PC. On-resonance variable delay multipulse scheme for imaging of fast-exchanging protons and semisolid macromolecules. Magn Reson Med 2017;77(2):730-739.
13. Xu X, Xu J, Chan KW, Liu J, Liu H, Li Y, Chen L, Liu G, van Zijl PC. GlucoCEST imaging with on-resonance variable delay multiple pulse (onVDMP) MRI. Magn Reson Med 2019;81(1):47-56.
14. Huang J, van Zijl PC, Han X, Dong CM, Cheng GW, Tse K-H, Knutsson L, Chen L, Lai JH, Wu EX, Xu J, Chan KW. Altered d-glucose in brain parenchyma and cerebrospinal fluid of early Alzheimer’s disease detected by dynamic glucose-enhanced MRI. Sci Adv 2020;6(20):eaba3884.
15. Chen L, Wei Z, Chan KW, Li Y, Suchal K, Bi S, Huang J, Xu X, Wong PC, Lu H. D-Glucose uptake and clearance in the tauopathy Alzheimer’s disease mouse brain detected by on-resonance variable delay multiple pulse MRI. J Cereb Blood Flow Metab 2020;DOI: 10.1177/0271678X20941264.
16. Gore J, Brown M, Mizumoto C, Armitage I. Influence of glycogen on water proton relaxation times. Magn Reson Med 1986;3(3):463-466.
17. Yadav NN, Xu J, Bar-Shir A, Qin Q, Chan KW, Grgac K, Li W, McMahon MT, van Zijl PC. Natural D-glucose as a biodegradable MRI relaxation agent. Magn Reson Med 2014;72(3):823-828.
18. Jin T, Mehrens H, Wang P, Kim S-G. Chemical exchange-sensitive spin-lock MRI of glucose analog 3-O-methyl-d-glucose in normal and ischemic brain. J Cerebr Blood F Met 2018;38(5):869-880.
19. Zu Z, Spear J, Li H, Xu J, Gore JC. Measurement of regional cerebral glucose uptake by magnetic resonance spin-lock imaging. Magn Reson Imaging 2014;32(9):1078-1084.
20. Jin T, Mehrens H, Hendrich KS, Kim S-G. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J Cerebr Blood F Met 2014;34(8):1402-1410.
21. Chen L, Cao S, Koehler RC, van Zijl PC, Xu J. High‐sensitivity CEST mapping using a spatiotemporal correlation‐enhanced method. Magn Reson Med 2020;84(6):3342-3350.
22. Hamy V, Dikaios N, Punwani S, Melbourne A, Latifoltojar A, Makanyanga J, Chouhan M, Helbren E, Menys A, Taylor S. Respiratory motion correction in dynamic MRI using robust data decomposition registration–Application to DCE-MRI. Med Image Anal 2014;18(2):301-313.
23. Melbourne A, Hipwell J, Modat M, Mertzanidou T, Huisman H, Ourselin S, Hawkes D. The effect of motion correction on pharmacokinetic parameter estimation in dynamic-contrast-enhanced MRI. Phys Med Biol 2011;56(24):7693.
24. Santarelli MF, Positano V, Giovannetti G, Frijia F, Menichetti L, Ardenkjaer-Larsen J-H, De Marchi D, Lionetti V, Aquaro G, Lombardi M. How the signal-to-noise ratio influences hyperpolarized 13C dynamic MRS data fitting and parameter estimation. NMR Biomed 2012;25(7):925-934.
Figures