0432
fMRI connectivity mapping in the awake mouse brain reveals state-dependent network reconfiguration1Functional Neuroimaging Laboratory, Istituto Italiano di Tecnologia, Rovereto, Italy, 2Ugo Basile S.r.L., Gemonio, Italy
Synopsis
Resting state fMRI mapping in the mouse is typically carried out under light anesthesia, preventing a full characterization of how the ensuing functional architecture compares to awake conditions. Leveraging a novel protocol for fMRI connectivity mapping in awake mice, we provide a fine-grained description of the network structure and dynamic organization of brain-wide functional connectivity in this species. Notably, by comparing network features across brain states, we identify a robust set of state-dependent network changes, including a distinctive dynamic signature of consciousness. These results open the way to the implementation of awake rsfMRI in the mouse.
Introduction
Recent years have seen an increased interest in the application of resting state fMRI (rsfMRI) in physiologically accessible species [1-3]. The application of these methods in the mouse has highlighted encouraging cross-species correspondences in the organization of functional networks [2, 4] offering novel opportunities to mechanistically probe the neural basis of rsfMRI network disruption in human brain disorders [5-7]. The vast majority of mouse rsfMRI studies to date have been carried out using light anaesthesia to induce immobilization during image acquisition [1, 8]. Although light anaesthesia has been convincingly shown to preserve fundamental topographic [9] and dynamic features of rsfMRI [10], sedative agents can produce unwanted genetic or pharmacological interactions [11], hence hampering the otherwise prominent mechanistic potential of rsfMRI mapping in rodents. More importantly, the lack of well-characterized rsfMRI datasets in awake mice prevents a full understanding of how light sedation affects the ensuing functional architecture compared to that obtained in conscious animals. This area of research is of special importance, given the increasing interest in the identification and characterization of potential rsfMRI signatures of consciousness across mammalian species [12, 13]. Here we describe a simple protocol enabling the acquisition of high quality rsfMRI timeseries in awake mice. We next provide a fine-grained description of the functional topography and dynamic structure of rsfMRI connectivity in the awake mouse brain. Notably, a comparison of awake features with those obtained under anaesthesia revealed a robust set of state-dependent network changes, including a distinctive dynamic signature of consciousness, encompassing the competing engagement of visual, basal forebrain and default-mode network areas that was characteristic of the awake state.Methods
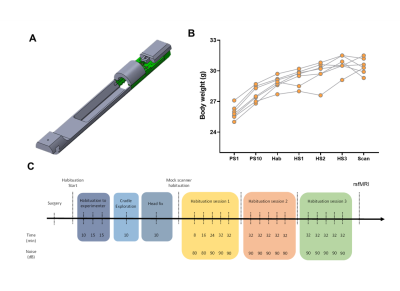
Awake mouse scanning was carried out in N=19 adult male C57Bl6/j mice. Custom-made head posts (Ugo Basile, Italy) were surgically implanted and mice were habituated to immobilization onto a custom-made animal cradle (Ugo Basile, Italy) as depicted in Fig. 1. rsfMRI scans acquired in n= 19 age-matched male C57Bl6/j mice under light anaesthesia (halothane 0.75%) were used as comparisons. These scans were previously acquired in our lab using the same hardware and imaging conditions of the awake study and are described in [10]. rsfMRI data were acquired at 7.0 Tesla with a 72mm birdcage transmit coil and a four channel solenoid coil for signal reception [10] using a single shot EPI sequence: TR/TE 1000/15 ms, flip angle 60°, matrix 98 x 98, FOV 2.3 x 2.3 cm, 18 coronal slices, slice thickness 550 µm, 32 min. Data preprocessing encompassed the following steps [14]: despiking, motion correction, skull stripping, registration, regression of CSF, white matter and motion related traces, band pass filtering, spatial smoothing, and motion censoring exceeding a displacement threshold of 0.75. To assess structure-function relationship [9] as a function of brain state, we calculated the strength of system segregation i.e. the relative strength of within-network connectivity compared to between-network connectivity as in [15]. Brain dynamics was assessed via frame-wise clustering to identify whole brain patterns of co-activation as in [10].Results and Discussion
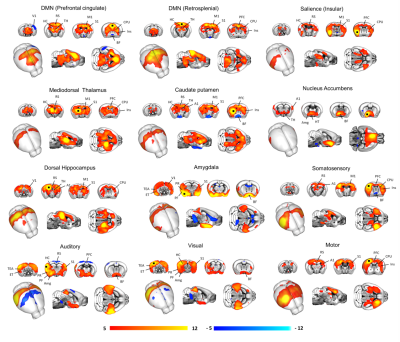
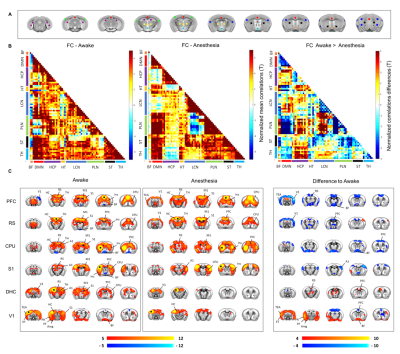
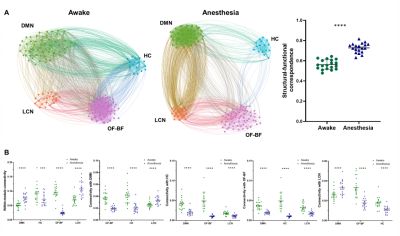
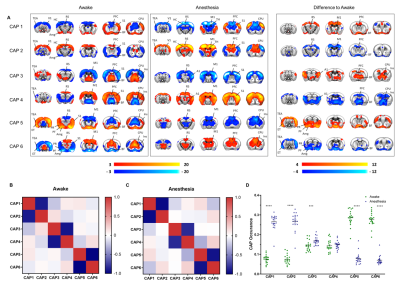
A schematic of the habituation procedure is depicted in Fig. 1. We opted for a gentle and extended habituation procedure to reduce restraint-induced stress. Seed based mapping (Fig. 2) revealed clear contralateral homotopic rsfMRI network connectivity, together with the presence of distributed networks previously characterized in anesthetized conditions, including an antero-posterior default mode network (DMN), a salience (insular-cingulate) network. Interestingly, a comparison of the obtained networks with the corresponding topography obtained under light anesthesia revealed a number of notable differences (Fig. 3). These include a generalized increase in between-network connectivity, and reduction in within-network rsfMRI connectivity under awake conditions. Moreover, prominent anticorrelation between DMN and visual/auditory areas was apparent in awake but not in anesthetized mice, leading to a segregation of these systems. A comparison between functional and axonal connectivity [9] corroborated this notion, revealing in the case of awake brain a greater crosstalk between networks, and significantly lower structural-functional correspondence. Prompted by the recent identification of dynamic signatures of consciousness in primates and human [12, 13], we hypothesized that these rsfMRI network changes could reflect a state-dependent underlying dynamic structure. To probe this hypothesis, we decomposed rsfMRI activity into recurring co-activation patterns (CAPs), as these have been recently shown to govern rsfMRI dynamics in the mammalian brain [10]. These analyses revealed that rsfMRI timeseries was composed by six dominant CAPs in both states (Fig. 5). Notably, occurrence of CAPs 1 and 2 was highly prominent in anaesthesia, while CAPs 5 and 6 were substantially more frequent in the awake state. Moreover, these occurrence differences were accompanied by focal changes in CAP topography that was characteristic of the awake state, the most prominent of which entailed a dramatic involvement of basal forebrain in awake CAPs, and anti-coordinated engagement of these substrate and visual-hippocampal areas with that default-mode network, hence recapitulating analogous rsfMRI dynamic signatures in human and primates [12, 13].Conclusions
Our results reveal state-dependent rsfMRI network reconfiguration in the awake mouse brain and highlight a possible dynamic signature of consciousness in this species.Acknowledgements
This study was supported by the European Research Council (ERC-DISCONN; no. 802371 to A.G.).References
1. Grandjean, J., et al., Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. 2019, Cold Spring Harbor Laboratory.
2. Gozzi, A. and A.J. Schwarz, Large-scale functional connectivity networks in the rodent brain. Neuroimage, 2016. 127: p. 496-509.
3. Milham, M., et al., Accelerating the Evolution of Nonhuman Primate Neuroimaging. Neuron, 2020. 105(4): p. 600-603.
4. Whitesell, J.D., et al., Regional, layer, and cell-class specific connectivity of the mouse default mode network. Neuron, 2020: p. 2020.05.13.094458.
5. Bertero, A., et al., Autism-associated 16p11.2 microdeletion impairs prefrontal functional connectivity in mouse and human BRAIN, 2018. 141(7): p. 2055–2065.
6. Pagani, M., et al., A cross-species link between mTOR hyperactivty and functional overconnectivity in autism in preparation.
7. Canella, C., et al., Cortical silencing results in paradoxical fMRI overconnectivity. bioRxiv, 2020: p. 2020.08.05.237958.
8. Sforazzini, F., et al., Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage, 2014. 87: p. 403-15.
9. Coletta, L., et al., Network structure of the mouse brain connectome with voxel resolution. Science Advances, 2020: p. 2020.03.06.973164.
10. Gutierrez-Barragan, D., et al., Infraslow State Fluctuations Govern Spontaneous fMRI Network Dynamics. Current Biology, 2019. 29(14): p. 2295-2306.e5.
11. Gozzi, A., et al., Drug-anaesthetic interaction in phMRI: the case of the pyschotomimetic agent phencyclidine. Magn Reson Imag, 2008. 26(7): p. 999-1006.
12. Demertzi, A., et al., Human consciousness is supported by dynamic complex patterns of brain signal coordination. Science Advances, 2019. 5(2): p. eaat7603.
13. Barttfeld, P., et al., Signature of consciousness in the dynamics of resting-state brain activity. Proceedings of the National Academy of Sciences, 2015. 112(3): p. 887-892.
14. Grandjean, J., et al., Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. bioRxiv, 2019: p. 541060.
15. Cohen, J.R. and M. D'Esposito, The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. The Journal of Neuroscience, 2016. 36(48): p. 12083-12094.
Figures