0429
Optogenetic fMRI Reveals Distinct Response Characteristics of Sensory and Limbic Thalamic Spindle-like Activities1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China
Synopsis
Spindle-like activities constitute one of the most critical brain-wide oscillatory activities for memory consolidation. Spindle-like activities with different temporal characteristics have been associated with heterogeneous distribution patterns. Studies postulated that such heterogeneous distribution and differences in temporal characteristics of spindle-like activities were determined by differences in corresponding spindle-generation thalamo-cortical circuits. However, no direct evidence has been shown. In this study, we demonstrate distinct brain-wide targets but similar temporal-characteristics dependent cross-modal recruitment property of limbic and sensory thalamically-evoked spindle-like activities. Our work provides direct evidence that spindle-like activities initiated from distinct thalamic nuclei can recruit distinct brain-wide targets
Purpose
Thalamo-cortical spindle-like activities constitute one of the major oscillatory activities in the brain and are critical for brain functions such as memory consolidation1-4. Such spindle-like activities are 7-14 Hz, 0.5-3 s brief oscillatory events that exhibit well recognizable spindle-shaped signal envelopes in their electroencephalogram (EEG) or local field potential (LFP) waveforms4,5. Spindle-like activities can initiate in a single local thalamo-cortical circuit and engage remote targets subsequently4,6. Over the past decades, numerous EEG7-9 and EEG activity-triggered fMRI10-14 studies have documented the heterogeneous distribution of distinct spindle-like activities associated with different temporal characteristics. Elucidating the roles played by distinct thalamic nuclei in shaping spatiotemporal characteristics of brain-wide spindle-like activities is critical to understand how distinct local spindle-like activities recruit their specific brain-wide targets15,16. However, progress has been hindered by the lack of specificity in neuroimaging studies of spindle-like activities, e.g., the EEG activity-triggered fMRI studies assuming the same HRF for spindle-like activities at all regions. In this study, we combine spatiotemporal precise optogenetic stimulation with whole-brain field of view fMRI to examine the brain-wide response characteristics of limbic versus sensory thalamically initiated spindle-like activities.Method
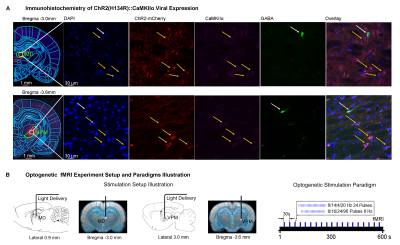
Animal preparation and MRI experimental setup: 3μl AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to medial dorsal (MD) and ventral posteromedial (VPM) thalamus of adult SD rats. After 4 weeks (Figure 1A), opaque optical fiber cannulas were implanted at the injection sites. All fMRI experiments were performed under 1.0% isoflurane. MRI data was acquired at 7T using GE-EPI.Optogenetic fMRI and electrophysiology experiments: 8, 16, 24 and 96 pulses 8Hz or 24 pulses 4, 14, and 20Hz of blue (473nm) light optogenetic stimulation were presented every 30s (10ms pulse width, 40mW/mm2; Figure 1B). Coherence analysis17 was applied to identify significant BOLD responses. BOLD profiles were extracted from atlas-based ROIs. To verify whether the brain-wide BOLD responses were evoked by spindle-like neural activities, local field potential (LFP) data (Figure 3A) was acquired using a multi-depth electrode (16 channels) at primary somatosensory barrel field (S1BF) and 5 single electrodes at VPM, amygdala (Amg), retrosplenial (RS), medial prefrontal cortex (mPFC) and superior colliculus (SC).
Results
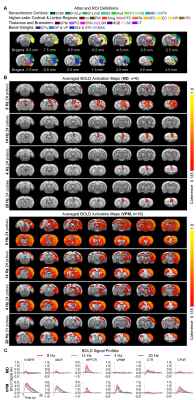
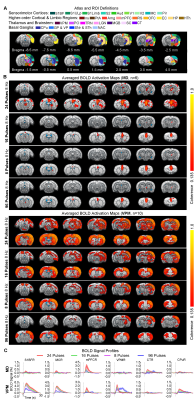
BOLD activations reveal distinct brain-wide targets for limbic versus somatosensory thalamically-evoked activities (Figure 2): We found that 8 Hz 24 pulses stimulation of MD recruited both limbic (central thalamus: CT; medial and orbital prefrontal cortices: mPFC and OFC; and RS) and sensorimotor cortical (primary somatosensory: S1BF; -limb region, S1Limb; -upper lip region, S1ULp; secondary somatosensory: S2; primary & secondary visual: V1 & V2; auditory: Aud; motor: MC; insular: Ins; parietal: PtA;), thalamic (visual: LGN), brainstem (SC) and striatal ( caudate putamen: CPu, pallidum: GP & VP) regions. BOLD activations at other stimulation frequencies (i.e., 14/4/20Hz) were confined within limbic regions. In comparison, 8 Hz 24 pulses stimulation of VPM recruited more sensorimotor cortical, thalamic, brainstem and basal ganglia regions (i.e., piriform, Pir; posterior nuclei, PO; auditory thalamus, MGB; thalamic reticular nucleus, TRN; nucleus accumbens, NAc) and limbic regions (Amg; hypothalamus, HTh; hippocampus, HP; entorhinal, EC) beyond the regions activated by MD stimulation, but the strongest activations were located in sensorimotor regions. However, varying the VPM stimulation frequency from 8 to 14/4/20Hz also resulted in decreases in cross-modal activations.MD and VPM-evoked BOLD activations exhibit a similar number of pulses-dependent response property (Figure 3): 24 pulses 8Hz optogenetic stimulation of MD and VPM evoked the most robust and widespread brain-wide BOLD activations. Changing the number of stimulation pulses from 24 to 16/8/96 resulted in weakened and confined responses.
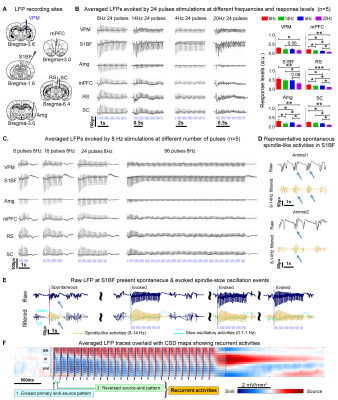
Multisite LFP recordings reveal VPM-evoked brain-wide cross-modal spindle-like activities (Figure 4): Robust spindle-like activities were evoked at all regions by the 8 Hz 24 pulses stimulation. However, with varying the stimulation frequency resulted in decreased LFP response levels and loss of spindle-shaped waveforms, especially in the remote regions such as Amg, mPFC, RS, and SC. Meanwhile, the prolonged stimulation (96 pulses) evoked weak sustained LFPs which lost spindle-shaped waveform in all regions
Discussion and Conclusion
Our study demonstrates that the optogenetic stimulation of MD, especially at 8 Hz 24 pulses, was able to recruit numerous brain-wide regions including sensorimotor-related thalamo-cortical and midbrain regions, limbic system, and basal-ganglia. The primary brain-wide targets of limbic and sensory thalamically initiated spindle-like activities are indeed different and correspond to distinct limbic and sensory thalamo-cortical circuits. Limbic thalamically initiated spindle-like activities recruit less brain-wide targets than sensory thalamically initiated spindle-like activities, corroborating recent evidence that sensory thalamo-cortical circuits are more engaged in spindle generation and synchronization18-21. However, some of the targets for sensory and limbic thalamically initiated spindle-like activities are shared at 24 pulses 8 Hz stimulation, indicating a robust brain-wide cross-modal recruitment property for spindle-like activities despite circuits of initiation. Furthermore, their frequency and number of stimulation pulses-dependent cross-modal recruitment property are similar. This indicates that despite different thalamo-cortical circuits, neural mechanisms underlying spindle generation, synchronization and termination are similar for limbic and sensory thalamic nuclei. Our LFP results confirmed that our evoked spindle-like activities from VPM were similar to spontaneous22,23 or optogenetically-evoked24,25 spindles. In conclusion, our study provides direct evidence that spindle-like activities initiated from different thalamic nuclei can recruit distinct brain-wide targets, and they can engage overlapping targets cross-modally when oscillating at slow spindle frequency and maximum spindle length.Acknowledgements
This study is supported in part by Hong Kong Research Grant Council (R7003-19, C7048-16G, HKU17112120, HKU17103819 and HKU17104020), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer’ Disease Diagnosis and Treatment (2018B030336001).References
Astori, S., Wimmer, R.D. & Luthi, A. Manipulating sleep spindles--expanding views on sleep, memory, and disease. Trends in Neurosciences 36, 738-748 (2013).
2. Luthi, A. Sleep Spindles: Where They Come From, What They Do. Neuroscientist 20, 243-256 (2014).
3. Antony, J.W., Schonauer, M., Staresina, B.P. & Cairney, S.A. Sleep Spindles and Memory Reprocessing. Trends Neurosci 42, 1-3 (2019).
4. Fernandez, L.M.J. & Luthi, A. Sleep Spindles: Mechanisms and Functions. Physiological Reviews 0, In Press (2019).
5. Warby, S.C., Wendt, S.L., Welinder, P., Munk, E.G., Carrillo, O., Sorensen, H.B., Jennum, P., Peppard, P.E., Perona, P. & Mignot, E. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods 11, 385-392 (2014).
6. Steriade, M., McCormick, D.A. & Sejnowski, T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679-685 (1993).
7. Contreras, D., Destexhe, A., Sejnowski, T.J. & Steriade, M. Spatiotemporal patterns of spindle oscillations in cortex and thalamus. Journal of Neuroscience 17, 1179-1196 (1997).
8. Purcell, S.M., Manoach, D.S., Demanuele, C., Cade, B.E., Mariani, S., Cox, R., Panagiotaropoulou, G., Saxena, R., Pan, J.Q., Smoller, J.W., Redline, S. & Stickgold, R. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun 8, 15930 (2017).
9. Dehghani, N., Cash, S.S. & Halgren, E. Emergence of synchronous EEG spindles from asynchronous MEG spindles. Hum Brain Mapp 32, 2217-2227 (2011).
10. Schabus, M., Dang-Vu, T.T., Albouy, G., Balteau, E., Boly, M., Carrier, J., Darsaud, A., Degueldre, C., Desseilles, M., Gais, S., Phillips, C., Rauchs, G., Schnakers, C., Sterpenich, V., Vandewalle, G., Luxen, A. & Maquet, P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A 104, 13164-13169 (2007).
11. Andrade, K.C., Spoormaker, V.I., Dresler, M., Wehrle, R., Holsboer, F., Samann, P.G. & Czisch, M. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci 31, 10331-10339 (2011).
12. Bergmann, T.O., Molle, M., Diedrichs, J., Born, J. & Siebner, H.R. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59, 2733-2742 (2012).
13. Fang, Z., Ray, L.B., Owen, A.M. & Fogel, S.M. Brain Activation Time-Locked to Sleep Spindles Associated With Human Cognitive Abilities. Front Neurosci 13, 46 (2019). 14. Laufs, H., Walker, M.C. & Lund, T.E. 'Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study'--its limitations and an alternative approach. Brain 130, e75; author reply e76 (2007).
15. Bastuji, H., Lamouroux, P., Villalba, M., Magnin, M. & Garcia-Larrea, L. Local sleep spindles in the human thalamus. J Physiol (2020).
16. Fernandez, L.M., Vantomme, G., Osorio-Forero, A., Cardis, R., Beard, E. & Luthi, A. Thalamic reticular control of local sleep in mouse sensory cortex. Elife 7(2018).
17. Lee, J.H., Durand, R., Gradinaru, V., Zhang, F., Goshen, I., Kim, D.S., Fenno, L.E., Ramakrishnan, C. & Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
18. Urbain, N., Fourcaud-Trocme, N., Laheux, S., Salin, P.A. & Gentet, L.J. Brain-State-Dependent Modulation of Neuronal Firing and Membrane Potential Dynamics in the Somatosensory Thalamus during Natural Sleep. Cell Rep 26, 1443-1457 e1445 (2019).
19. Hagler, D.J., Jr., Ulbert, I., Wittner, L., Eross, L., Madsen, J.R., Devinsky, O., Doyle, W., Fabo, D., Cash, S.S. & Halgren, E. Heterogeneous Origins of Human Sleep Spindles in Different Cortical Layers. J Neurosci 38, 3013-3025 (2018).
20. Sirota, A., Csicsvari, J., Buhl, D. & Buzsáki, G. Communication between neocortex and hippocampus during sleep in rodents. Proceedings of the National Academy of Sciences of the United States of America 100, 2065-2069 (2003).
21. Li, Y., Lopez-Huerta, V.G., Adiconis, X., Levandowski, K., Choi, S., Simmons, S.K., Arias-Garcia, M.A., Guo, B., Yao, A.Y., Blosser, T.R., Wimmer, R.D., Aida, T., Atamian, A., Naik, T., Sun, X., Bi, D., Malhotra, D., Hession, C.C., Shema, R., Gomes, M., Li, T., Hwang, E., Krol, A., Kowalczyk, M., Peca, J., Pan, G., Halassa, M.M., Levin, J.Z., Fu, Z. & Feng, G. Distinct subnetworks of the thalamic reticular nucleus. Nature 583, 819-824 (2020).
22. Coppieters 't Wallant, D., Maquet, P. & Phillips, C. Sleep Spindles as an Electrographic Element: Description and Automatic Detection Methods. Neural Plast 2016, 6783812 (2016).
23. Contreras, D. & Steriade, M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. The Journal of Physiology 490, 159-179 (1996).
24. Kim, A., Latchoumane, C., Lee, S., Kim, G.B., Cheong, E., Augustine, G.J. & Shin, H.S. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc Natl Acad Sci U S A 109, 20673-20678 (2012).
25. Latchoumane, C.-F., Ngo, H.-V., Born, J. & Shin, H.-S. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424-435 (2017).
26. McCormick, D.A., McGinley, M.J. & Salkoff, D.B. Brain state dependent activity in the cortex and thalamus. Current Opinion in Neurobiology 31, 133-140 (2015). 27. Contreras, D. & Steriade, M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. The Journal of physiology 490 ( Pt 1), 159-179 (1996).
Figures