0424
Motion-insensitive DTI of Kidney using Prospective Acquisition Motion Correction Triggering
Arun Joseph1,2,3, Laila-Yasmin Mani4, Tom Hilbert5,6,7, Thomas Benkert8, Tobias Kober5,6,7, Bruno Vogt4, and Peter Vermathen3
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, Sitem-Insel, Bern, Switzerland, 3Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland, 4Department of Nephrology and Hypertension, University Hospital Bern, Inselspital, Bern, Switzerland, 5Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 6Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 7LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 8Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Bern, Switzerland, 2Translational Imaging Center, Sitem-Insel, Bern, Switzerland, 3Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland, 4Department of Nephrology and Hypertension, University Hospital Bern, Inselspital, Bern, Switzerland, 5Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 6Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 7LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 8Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Diffusion tensor imaging (DTI) of the kidney provides important functional information such as diffusion and micro-perfusion of the tissue and additionally estimates anisotropic diffusion of water in renal tubuli. However, these measurements are highly sensitive to respiration-induced motion artifacts which bias the obtained functional information. Here, we propose to use prospective acquisition motion correction (PACE) in combination with free-breathing acquisitions for motion-insensitive diffusion measurements of the kidney. A preliminary qualitative and quantitative validation is performed on healthy subjects comparing results from conventional respiratory-triggered to PACE-triggered DTI.
Introduction
DTI of abdominal organs, in particular kidney, is promising as it provides both structural and functional information1,2. However, DTI in abdominal regions is challenging due to its high sensitivity to respiration-induced motion artifacts. In recent years, many approaches have been investigated to overcome these challenges, such as measuring during breathhold, during free-breathing with respiratory triggering or during free-breathing using retrospective image co-registration. Triggering using respiratory belts can be affected by inconsistent respiration, leading to residual motion artifacts; it may also suffer from improper belt positioning or movement while scanning, which may lead to prolonged or even interrupted scans.To address this problem, prospective acquisition motion correction (PACE) triggering is used which employs automatic detection of the different phases of the respiration cycle to acquire data and has been previously demonstrated for DTI of the kidney3. However, to our knowledge, a systematic comparison of renal DTI between conventional respiratory triggering and PACE triggering has not been performed. In this study, we implemented PACE trigger for DTI of the kidney and evaluated it with respiratory-triggered acquisitions on healthy subjects.
Methods
Ten healthy volunteers (6 female, 4 male, median age: 40y, range: 26–63y) were measured at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with 18-channel thorax and 32-channel spine receive coils in the anterior and posterior regions, respectively. A prototype diffusion-weighted (DW) single-shot echo-planar sequence was used which can perform measurements with both respiratory and PACE triggering. PACE triggering is a navigator-based technique based on a FLASH-based navigator to monitor the movement of the diaphragm4-7. The DTI acquisitions are performed according to the measured diaphragm position.A standard DW single-shot echo-planar sequence with conventional respiratory triggering was used as a reference. The final protocol consisted of three measurements - reference with respiratory triggering (SDTI_RT), prototype sequence with respiratory (PDTI_RT) and PACE triggering (PDTI_PACE). The measurements were performed with the following scan parameters: TRmin 2000 ms, TE 43 ms, spatial resolution of 1.42×1.42×1.42 mm3. Seven slices were acquired (thickness: 5mm gap: 6mm). At each slice position, 75 images were acquired with 7 different b-values 0-800s/mm2 in 6 non-collinear directions.
Co-registration of individual images per exam was performed prior to further processing using a multimodal non-rigid registration algorithm8. Mono- and biexponential fitting was performed, yielding the total ADCT and the perfusion cleared ADCD, respectively, the perfusion fraction FP, and the fractional anisotropy (FA). Regions of interest (ROI) were placed on several slices for each subject in medulla and cortex with total voxel-averages of 135±44 and 180±67 pixels, respectively. The ROIs were placed independently for the three different DTI measurements. The different modalities were compared in two ways as has been described before8: 1) For each analyzed ROI, the standard deviation (SDROI) was calculated from all pixels within the ROIs (assuming that the ROIs were placed on areas presenting homogeneous tissue and differences between variations within ROIs are due to motion); 2) The deviation from diffusion-model fitting was determined comparing the relative root mean squared error (RMSE).
Results and Discussion
The three different DTI measurements (SDTI_RT, PDTI_RT, PDTI_PACE) were performed successfully in all 10 subjects, except for one respiratory-triggered scan (PDTI_RT), which was aborted after a long period without trigger event. Measurement duration for SDTI_RT was 06:22±01:21min, which was significantly longer than for PDTI_PACE with 5:33±1:11min (p=0.006), but also significantly longer than PDTI_RT with 5:29±0:44min (p=0.014). The time difference may therefore partly be due to more efficient triggering of PACE versus respiratory triggering but may also be due to a learning effect of the volunteers.All measurements were included in the analysis. Parameter maps were obtained from the co-registered images. The ADCT, ADCD, FP, and FA maps demonstrated visually good quality (Fig.1). Quantitative analysis demonstrated slightly but significantly lower RMSE values for PDTI_PACE compared to both respiratory triggered scans in cortex and compared to PDTI_RT in medulla (Table 1). SDROI were lower for almost all parameters in the PACE compared to the respiratory triggered scans with some differences reaching significance (Table 2).
The derived parameters demonstrated relatively small variances for all three sequence variants and the values are in the range of previously published values9. The parameters were not significantly different between the PACE and respiration-triggered scans (Table 3). ADC and FP values were found to be lower in medulla than in cortex for all three modalities (significant for SDTI_RT and the PDTI_PACE) with greatest cortico-medullary differences for the PACE triggered scan. FA values were much higher in medulla than in cortex. These differences are in accordance with previous findings.
Conclusion
The results demonstrate robust performance and reliable results for the prototype DTI sequence with PACE triggering. Although also the respiratory-triggered scans performed well in these measurements on motivated healthy volunteers, the PACE triggering yielded slight, but significant improvement. Possibly more important however, the PACE-triggered sequence is expected to result in fewer interrupted DTI measurements in more challenging patient measurements (as was indicated by the single interrupted scan in the current study), because it does not depend on placing, adjusting, or potential movement of the respiratory belt. Thus, the current study demonstrates that PACE triggering can replace respiratory triggering in DTI.Acknowledgements
No acknowledgement found.References
- Notohamiprodjo M, Glaser C, Herrmann KA, et al. Diffusion tensor imaging of the kidney with parallel imaging: initial clinical experience. Invest Radiol. 2008; 43:677–685.
- Kataoka M, Kido A, Yamamoto A, et al. Diffusion tensor imaging of kidneys with respiratory triggering: optimization of parameters to demonstrate anisotropic structures on fraction anisotropy maps. Jour Magn Reason Imag. 2009; 29:736–744.
- Chana RW, Von Deuster C, Stoeckb CT, et al. High-resolution diffusion tensor imaging of the human kidneys using a free-breathing, multi-slice, targeted field of view approach. NMR Biomed. 2014; 27:1300–1312.
- Wang Y, Rossman PJ, Grimm RC, et al. Navigator-echo-based real-time respiratory gating and triggering for reduction of respiration effects in three-dimensional coronary MR angiography. Radiology 1996; 198:55-60.
- Stuber M, Botnar RM, Danias PG, et al. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology 1999; 212:579–587.
- Asbach P, Klessen C, Kroencke TJ, et al. Magnetic resonance cholangiopancreatography using a free-breathing T2-weighted turbo spin-echo sequence with navigator triggered prospective acquisition correction. Magn Reson Imaging 2005; 23:939–945.
- Zech CJ, Herrmann KA, Huber A, et al. High-Resolution MR-Imaging of the Liver with T2-weighted sequences using integrated parallel imaging: comparison of prospective motion correction and respiratory triggering. Jour Magn Reason Imag. 2004; 20:443–450.
- Lu H, Cattin PC, Reyes M. A hybrid multimodal non-rigid registration of MR images based on diffeomorphic demons. Conf Proc IEEE Eng Med Biol Soc 2010;2010: 5951–5954.
- Seif M, Lu H, Boesch C, Reyes M, Vermathen P. Image registration for triggered and non‐triggered DTI of the human kidney: Reduced variability of diffusion parameter estimation. J Magn Reson Imaging. 2015; 41: 1228–1235.
Figures
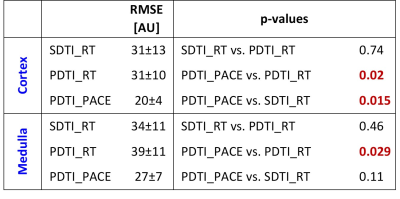
Table
1: RMSE values in medulla
and cortex obtained from the three DTI measurements and comparison between the
three sequence variants.
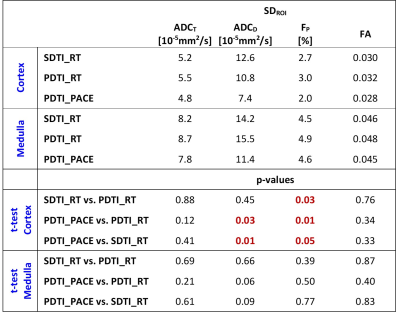
Table 2: Standard Deviations within ROI (SDROI) in
medulla and cortex obtained from the three DTI measurements and comparison
between the three sequence variants.
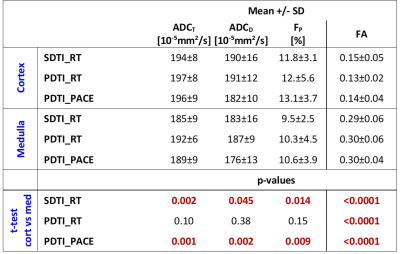
Table
3: Mean ± SD of the DTI
parameters obtained from the three different sequence variants and comparison
between medulla and cortex.
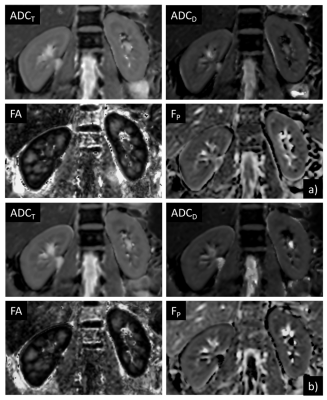
Figure 1: Example ADCT, ADCD, FA, and FP
parameter maps a) derived from a PACE-triggered and (b) derived from a
respiratory-triggered DTI scan of a volunteer.