0416
The Feasibility of Radiofrequency Rhizotomy Lesion Visualization in the Trigeminal Ganglion using 7.0-Tesla MRI1Center for Magnetic Resonance Imaging, University of Minnesota, Minneapolis, MN, United States, 2Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 3Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 4Department of Diagnostic and Biological Science, University of Minnesota, Minneapolis, MN, United States
Synopsis
Pain recurrence following invasive treatments for trigeminal neuralgia is common, yet the treatment decisions in these situations lack objective guidance. The goal of this work was to establish the feasibility of using 7.0-Tesla MRI to visualize treatment related effects from percutaneous radiofrequency rhizotomy procedures. We scanned two patients with trigeminal neuralgia and one human cadaver specimen at 7.0-Tesla after radiofrequency rhizotomy procedures. Both acute and long-term treatment related effects were successfully visualized by our protocol. Future work will recruit a large cohort of trigeminal neuralgia patients to correlate imaging findings with clinical outcomes.
Introduction
Trigeminal neuralgia (TN) is the most painful neuropathic condition. For a large portion of patients with TN, medication proves insufficient to control their pain. A number of percutaneous and surgical options exist for these patients1. One of the most popular options is percutaneous radiofrequency rhizotomy, a minimally invasive procedure where an electrode needle is navigated into the trigeminal ganglion and a thermocoagulative lesion is induced. However, pain recurrence rates for this procedure approach 50% at 5 years2,3. In these cases, the clinical decision between repeating the rhizotomy and proceeding with a different invasive technique lacks objective guidance. Our hypothesis is that a non-invasive method to visualize and characterize the induced lesion over time will help clinicians make treatment decisions that reduce the chance of pain recurrence or intolerable numbness. The purpose of this study is to assess the feasibility of using 7.0-Tesla MRI to detect and characterize small lesions induced by the rhizotomy procedure with in-vivo and ex-vivo experiments. Our long-term goal is to expand our methods to a large cohort of trigeminal neuralgia patients and correlate image based findings with clinical outcomes.Methods
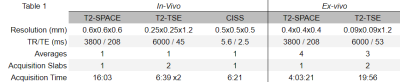
In-vivo: patients with TN were recruited for 7.0-Tesla imaging. The protocol consisted of T1-MPRAGE, T2-SPACE, CISS, and T2-TSE imaging4. Two patients were included in this analysis to compare the effects of different rhizotomy treatments. The first case was a surgically naive TN patient undergoing a radiofrequency rhizotomy procedure. This patient was scanned two weeks prior to their procedure, and three days following the successful procedure using the same protocol at 7.0-Tesla. The second case was a TN patient who had four prior failed radiofrequency rhizotomy procedures, the most recent performed one year prior to imaging. This patient was scanned once.Ex-vivo: An intact, unpreserved, whole human cadaver specimen was acquired for imaging. At first, the specimen was scanned at 7.0-Tesla. Sequences used in-vivo were adapted to allow for higher resolution and averaging. Following the initial scan, the specimen was moved to a CT scanner, located in the same facility, where a percutaneous radiofrequency rhizotomy was performed by a neurosurgeon on both trigeminal nerves, using CT guidance. The specimen was returned to the 7.0-Tesla scanner where the initial MR protocol was acquired a second time. The specifications for the sequences used both in-vivo and ex-vivo are displayed in table 1.
Analysis: T2-TSE was acquired in two slabs of interleaved slices. These were registered and combined using an in-house algorithm5. For the subjects scanned twice, the T2-space and T2-TSE image volumes were first histogram-matched and then registered using a consecutive affine then b-spline registration algorithm. The difference between the pre- and post- procedure image volumes was calculated for comparison and for signal change detection.
Results
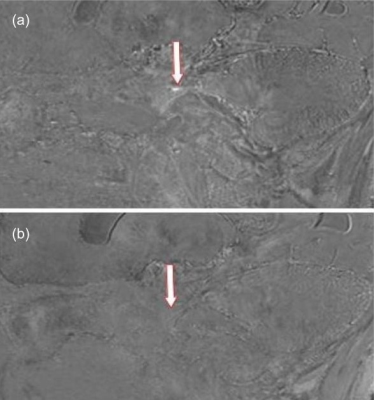
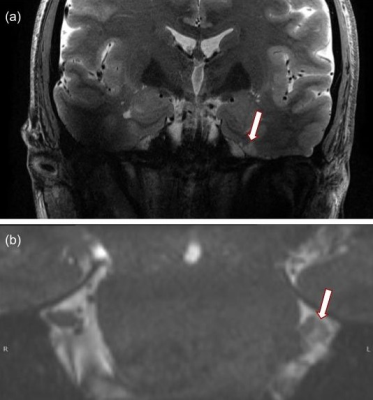
All 7T MR images were deemed of high quality, with a high level of similarity before and after surgery, both in vivo and ex vivo. In-vivo: Post-operative changes were observed in both patients. In the patient scanned 3 days following their initial rhizotomy procedure, a focal hypointense lesion was observed in the trigeminal ganglion on both the T2-SPACE and T2-TSE imaging (figure 1). No other structural changes to the trigeminal nerve were observed. In the patient scanned following several rhizotomy procedures, broad nerve atrophy was observed in the trigeminal nerve root and the nerve rootlets in Meckel’s cave on the CISS imaging (figure 2). Further, mild encephalomalacia was observed on the medial aspect of the temporal lobe against the wall of Meckel’s cave. Ex-vivo: Thanks to the complete absence of physiological motion, MRI images, devoid of commonly observed related artifacts, preserved very sharp anatomical details (figure 3). Numerous, broad T2 hypointensities in Meckel’s cave, surrounded with irregular hyperintensities, were observed post-procedurally that were not present pre-procedure.Discussion
The hypointense lesion observed in the patient scanned before and immediately following the procedure suggests that 7.0-Tesla imaging has the necessary sensitivity to detect early thermocoagulative lesions in the ganglion. In the patient with multiple procedures the widespread atrophy observed in Meckel’s cave was expected while the atrophy of the cisternal and root entry zone segments of the nerve suggests that the induced nerve injury can have wider effects to nerve anatomy beyond the initial lesion6. The human specimen scanned establishes excellent resolution and contrast in Meckel’s cave for lesion observation. However, the post-procedure scan revealed widespread hypointensities highly suggestive of susceptibility artifacts. These may be due to: a) air introduced during the procedure, b) treatment related effects or c) gas formation in the ganglion post-procedure. Further experimentation is required to confirm these findings and investigate methodological remedies.Conclusion
This work establishes the feasibility of observing short- and long-term percutaneous radiofrequency rhizotomy related treatment effects in the trigeminal ganglion. Future work will recruit more patients and perform more experiments in unpreserved human specimens to correlate findings with clinical outcomes and to characterize lesions based on the underlying anatomical and physiological changes.Acknowledgements
This research was supported by NIBIB P41 EB027061 and P30 NS076408. Personnel performing this research were also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grants TL1R002493 and UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.References
1. Guo H, Song G, Wang X, Bao Y. Surgical treatment of trigeminal neuralgia with no neurovascular compression: A retrospective study and literature review. J Clin Neurosci. 2018;58:42-48.
2. Ho K-Y, Emril. Treatment of trigeminal neuralgia: role of radiofrequency ablation. J Pain Res. Published online 12/2010:249.
3. Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous Controlled Radiofrequency Trigeminal Rhizotomy for the Treatment of Idiopathic Trigeminal Neuralgia: 25-year Experience with 1600 Patients. Neurosurgery. 2001;48(3):524-534.
4. Gizewski ER, Maderwald S, Linn J, et al. High-resolution anatomy of the human brain stem using 7-T MRI: improved detection of inner structures and nerves? Neuroradiology. 2014;56(3):177-186.
5. Henry TR, Chupin M, Lehéricy S, et al. Hippocampal sclerosis in temporal lobe epilepsy: findings at 7 T1. Radiology. 2011;261(1):199-209.
6. Northcutt BG, Seeburg DP, Shin J, et al. High-Resolution MRI Findings following Trigeminal Rhizotomy. AJNR Am J Neuroradiol. 10/2016;37(10):1920-1924.
Figures