0409
Enhancement of PNS risk in the presence of a metallic knee prosthesis1Istituto Nazionale di Ricerca Metrologica, Torino, Italy, 2Dipartimento Ingegneria Industriale, Università degli Studi di Padova, Padova, Italy, 3Istituto Ortopedico Rizzoli, Bologna, Italy
Synopsis
Peripheral nerve stimulation is a typical source of concern in MRI, which, in principle, could be significantly affected by the presence of a foreign object, like an orthopaedic prosthesis, in the body. This work investigates this possibility through a computational approach, making use of a human model where a knee implant has been placed, realistically, through a “virtual surgery”. Results indicate that the presence of the prosthesis can give rise to local enhancement of the electric field induced by the operation of the gradient coils, hence increasing the risk.
INTRODUCTION
Peripheral nerve stimulation (PNS) is the sensation of an activation of the nervous system induced by low-frequency magnetic fields. In MRI, PNS can occur due to gradient fields and represents a safety concern because it may be uncomfortable, or even intolerably painful, for the patient. A number of scientific articles1-8 have already discussed the problem, but, so far, no paper has addressed the investigation of PNS for patients carrying a prosthesis, whose presence could modify the distribution of the induced electric field. This work provides a first evaluation of the possible enhancement of the electric field induced around a knee prosthesis (i.e. one of the most prevalent orthopaedic implants) with respect to the field that would take place without it. The analysis is carried out through numerical simulations, applied to an anatomical human model including a realistic knee implant.METHODS
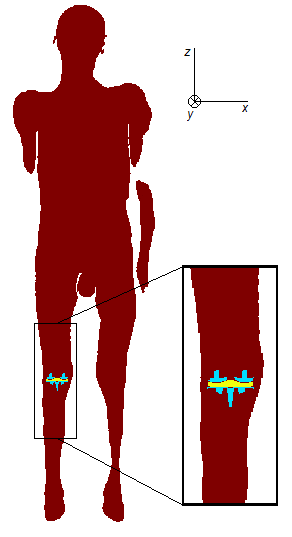
The investigation is performed on the anatomical model “Glenn”9, whose tissue properties are characterized according to10. A “virtual surgery” has been applied to the left leg of the model, to implant the knee prosthesis. The latter comprises the tibial and femoral components, both made of a CoCrMo alloy (conductivity: 1.16 MS/m) and an insulating liner (Fig. 1). To reproduce what happens in a real surgery, some pieces of the bones have been cut away, obtaining some voids where the implant does not replace the removed tissues perfectly. In reality, such voids would be filled by the synovial fluid and the re-arrangement of the soft tissues. Since the adopted database of tissue conductivities does not include the synovial fluid, the conductivity of the cerebrospinal fluid has been used for the filler.The body is exposed to a homogeneous, unit, magnetic flux density, directed along the x, or y, or z axes, which correspond to the left-right, posterior-anterior and feet-head directions, respectively. The simulations are performed under sinusoidal time behavior, at 100 Hz or 1000 Hz, segmenting the models into 2 mm cubic voxels and using a homemade code based on a Volume Integral Equation method, accelerated by the FFT transform and Algebraic Multigrid Preconditioner, able to take into account that the metallic objects perturb the magnetic field.
The exposure is evaluated in a region around the knee, with a z-extent of 38 cm. When analyzing the results for the implanted model, the voxels belonging to the prosthesis and the filler are ignored. The corresponding voxels are discarded also from the results of the model without implant. Following the prescriptions given in standard IEC 60601-2-3311, i.e. adopting a rheobase = 2.2 V/m, a chronaxie = 0.36 ms and the proper effective stimulus duration (which, for sinusoidal signals, is equal to 3.2 ms at 100 Hz and 0.32 ms at 1000 Hz), in “normal operating mode” the MRI scanner should not induce an electric field larger than 1.96 V/m and 3.74 V/m, at 100 Hz and 1000 Hz respectively, to avoid triggering the PNS (with a 20 % safety factor). These values are used here as a reference.
RESULTS
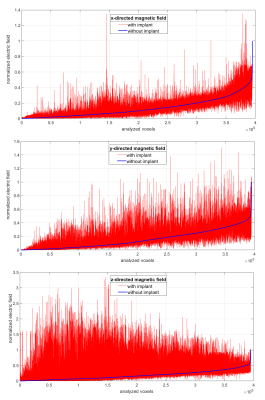
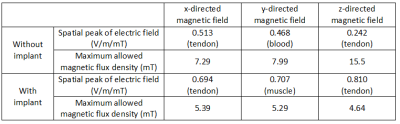
The electric field induced at 1000 Hz in the region of interest is reported in Fig. 2. In order to put in evidence both the absolute and relative discrepancies, the results have been sorted in ascending order with reference to the situation without implant and normalized to its maximum value. The normalised results at 100 Hz are almost coincident to those at 1000 Hz and therefore not reported (in tissues, the field is almost proportional to frequency). Table 1 provides the spatial peak of the electric field corresponding to an applied magnetic flux density of 1 mT; the tissue where such a value is found is indicated as well. The same table shows the magnetic flux density that would be needed to reach the limit of electric field recommended by IEC 60601-2-33. To provide a visual inspection, Fig. 3 reports the field on a sagittal section, for the case that maximizes the enhancement of the electric field (i.e. z-directed magnetic field).DISCUSSION AND CONCLUSIONS
In the presence of the implant, a significant number of voxels exhibit a visible change (reduction or enhancement) of the induced electric field. In all cases, the spatial peak gets worse with the implant, especially for the z-oriented magnetic field. A magnetic flux density of a few millitesla (a value that would be easily found in correspondence of the knee, when imaging the pelvis with cylindrical MRI scanners) is enough to rescale the electric field to the allowed limit at 1000 Hz, suggesting a potential risk. At 100 Hz the risk is lower, because the threshold is almost halved, but the induced electric field is 10 times weaker.This analysis must be seen as preliminary because, in its current form, it suffers some limitations. Among them, it is worth mentioning the use of a homogeneous magnetic field and the absence of a detailed network of nerves in the model, which forces to monitor the electric field in all tissues, without distinctions. Despite this, based on the discussed outcomes, the presence of a metallic implant should be considered as an aggravating circumstance when dealing with PNS.
Acknowledgements
The results here presented have been developed in the framework of the EMPIR Project 17IND01 MIMAS. This project 17IND01 MIMAS has received funding from the EMPIR programme co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme.
The authors acknowledge the support of Zurich MedTech in the development of the implanted human model.
References
- Ham CLG, Engels JML, vandeWiel GT, Machielsen A. Peripheral nerve stimulation during MRI: Effects of high gradient amplitudes and switching rates. Journal of Magnetic Resonance Imaging 1997;7:933-937.
- Ehrhardt JC, Lin CS, Magnotta VA, Fisher DJ, Yuh, WTC. Peripheral nerve stimulation in a whole-body echo-planar imaging system. Journal of Magnetic Resonance Imaging 1997;7:405-409.
- Den Boer JA, Bourland JD, Nyenhuis JA, Ham CLG, Engels JML, Hebrank FX, Frese G., Schaefer DJ. Comparison of the Threshold for Peripheral Nerve Stimulation During Gradient Switching in Whole Body MR Systems. Journal of Magnetic Resonance Imaging 2002;15:520–525.
- Liu F, Zhao H, Crozier S. On the Induced Electric Field Gradients in the Human Body for Magnetic Stimulation by Gradient Coils in MRI. IEEE Transactions on Biomedical Engineering 2003;50:804-815.
- So PPM, Stuchly MA, Nyenhuis JA. Peripheral Nerve Stimulation by Gradient Switching Fields in Magnetic Resonance Imaging. IEEE Transactions on Biomedical Engineering 2004;51:1907-1914.
- Samoudi AM, Vermeeren G, Tanghe E, Van Holen R, Martens L, Joseph W. Numerically simulated exposure of children and adults to pulsed gradient fields in MRI. Journal of Magnetic Resonance Imaging 2016;44:1360–1367.
- Klein V, Davidea M, Wald LL, Schad LR, Guerin B. Sensitivity analysis of neurodynamic and electromagnetic simulation parameters for robust prediction of peripheral nerve stimulation. Physics in Medicine and Biology 2019;64:015005.
- Davids M, Guerin B, vom Endt, A Schad, LR, Wald LL. Prediction of peripheral nerve stimulation thresholds of MRI gradient coils using coupled electromagnetic and neurodynamic simulations. Magnetic Resonance in Medicine 2019;81:686-701.
- IT’IS Foundation, Virtual Population. https://itis.swiss/virtual-population/virtual-population/overview/. Accessed December 15, 2020.
- IT’IS Foundation, Low frequency (Conductivity). https://itis.swiss/virtual-population/tissue-properties/database/low-frequency-conductivity/. Accessed December 15, 2020.
- International Electrotechnical Commission (IEC) IEC 60601-2-33:2010 +AMD1:2013+AMD2:2015: Medical electrical equipment - particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis, Edition 3.2. IEC, Geneva, 2015.
Figures