0385
Cardiac disease may exacerbate age-related white matter disruptions: improvements are feasible after cardiac rehabilitation1Lawson Imaging, Lawson Health Research Institute, London, ON, Canada, 2Medical Biophysics, Western University, London, ON, Canada, 3Cardiology, Western University, London, ON, Canada, 4School of Kinesiology, Western University, London, ON, Canada, 5Clinical Neurological Sciences, Western University, London, ON, Canada, 6Research Centre for Studies in Aging, McGill University, Montreal, QC, Canada
Synopsis
White matter (WM) degeneration is associated with cognitive impairment in coronary artery disease (CAD). We used diffusion tensor imaging to assess WM integrity in brains of CAD patients before and after cardiac rehabilitation (CR) and in young and old healthy controls (HC). Widespread WM changes were observed between older and younger HC, while robust WM changes were observed in WM regions linked to cognition in CAD patients at baseline with improvements following CR. In CAD, disease manifestation and brain aging may contribute to changes in brain WM macrostructure with potential influence on cognition, and these may be quelled by CR.
INTRODUCTION
In coronary artery disease (CAD), damage to the brain’s white matter (WM) has been associated with cognitive impairment and decreased quality of life.1 These WM alterations may occur over time as a result of disease-associated changes, age-related effects, or a combination of both. Advanced MRI techniques, such as diffusion tensor imaging (DTI), can non-invasively characterize WM macrostructure in-vivo by measuring water diffusion in the brain.2 DTI scalars fractional anisotropy (FA), mean diffusivity (MD), and radial diffusivity (RD) have been shown to provide indirect quantitative measures of WM fiber integrity.3 Specifically, decreases in FA can reveal WM macrostructural breakdown in the brain4 while increases in MD and RD may indicate edema, axonal degeneration or demyelination.5 Recent studies have shown changes in FA to be closely associated with executive function in CAD1 as well as age.6 In this study, we used DTI indices to assess disease-related and age-related effects on WM macrostructure integrity in the brains of CAD patients who were previously found to have brain atrophy, hypoperfusion, and low cognitive performance.7,8 WM integrity was also assessed in CAD patients following a 6-month cardiac rehabilitation (CR) program to see whether CR improves WM macrostructure in CAD.METHODS
A 10-min diffusion-weighted MRI scan was performed on 35 CAD patients (ten females; mean age = 58 ± 8 yr), 21 age-matched healthy controls (HC) (nine females; mean age = 58 ± 9 yr) and 25 younger HC subjects (14 females; mean age = 26 ± 4 yr) using a Siemens 3T Verio scanner. A subset of the CAD cohort (n=19) was rescanned 6-months after completing a low-to-moderate aerobic exercise-based CR program. Diffusion-weighted imaging acquisition protocol: single-shot EPI with 64 diffusion encoding directions; b-values = 0 and 1000 s/mm2; 2 mm3 isotropic voxels. Images were denoised using an optimized non-local means filter9–11 in MATLAB (MathWorks®, Natick, MA). Gibbs ringing artifact removal was performed using the local subvoxel shifts algorithm12 in MRtrix3.13 Subject motion and eddy current corrections were performed using FSL14 and MRtrix3. Bias field correction was completed using the N4 algorithm in ANTS.15 Diffusion tensors were fit to the data using non-linear least-squares estimation in ExploreDTI16 to generate FA, MD, and RD maps. DTI scalar maps were spatially normalized to the MNI space using ANTS. Voxel-wise tract-based spatial statistics (TBSS) was performed in FSL to compare DTI indices between: CAD patients at baseline and age-matched HC subjects (disease-related effect); older and younger HC subjects (age-related effect); and CAD patients pre-CR and post-CR (CR-related effect). For TBSS statistical analyses, p < 0.05 (FWE-corrected) was considered statistically significant.RESULTS
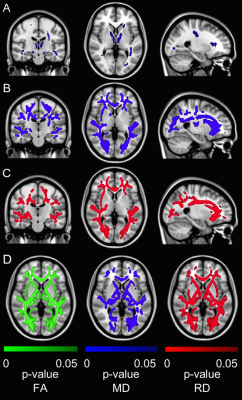
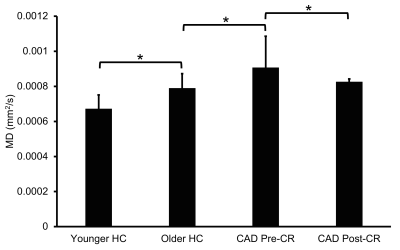
Compared to age-matched controls, CAD patients at baseline had increased MD in the longitudinal fasciculus, thalamic radiation, and corticospinal tract (Fig. 1A). Older HC subjects had widespread increases in MD (Fig. 1B) and RD (Fig. 1C) throughout brain WM compared to younger HC subjects. Increased FA, decreased MD, and decreased RD were also widespread throughout brain WM in CAD patients after CR, including several WM regions linked to cognitive function (Fig. 1D), suggesting WM integrity improved following CR. Across all subjects, group mean MD was increased in CAD patients at baseline relative to controls with an 8.9% recovery in MD observed in CAD patients following CR (Fig. 2).DISCUSSION
In this study, we non-invasively assessed WM integrity in CAD patients (at baseline and following CR) and healthy adults to investigate disease-related, age-related, and CR-related effects on WM macrostructure in CAD. We observed WM changes in CAD patients at baseline compared to age-matched controls as well as in older controls compared to younger controls, suggesting that both disease manifestation and brain aging may be contributing factors to WM dysfunction and WM-associated cognitive decline in CAD. More importantly, we observed robust improvements in WM integrity, especially in WM pathways linked to cognition, in CAD patients following CR, suggesting CR may have a neuroprotective effect against WM degeneration in CAD. In the future, we will use diffusion MRI tractography and region-based analyses of DTI indices to further assess WM macrostructural changes in CAD.CONCLUSION
In general, DTI can be a useful tool for non-invasive in-vivo characterization of WM macrostructure in CAD. Brain aging may be a contributing factor to WM degeneration in CAD. CR can improve WM integrity in the brain and may be a useful intervention strategy for quelling WM and mind degenerations in CAD. Future studies will use diffusion MRI tractography to further explore WM macrostructure changes in CAD.Acknowledgements
This work was supported by funding from the Heart and Stroke Foundation of Ontario (JKS and UCA) and from Western University Department of Medicine Program of Experimental Medicine (NS). The authors thank Tim Hartley for his role in coordination of the study, John Butler for assistance with MR imaging and Madeleine Dacey for initial preliminary analysis of the data.References
1. Santiago C, Herrmann N, Swardfager W, et al. White Matter Microstructural Integrity Is Associated with Executive Function and Processing Speed in Older Adults with Coronary Artery Disease. The American Journal of Geriatric Psychiatry 2015;23:754–63.2.
2. Mori S, Zhang J. Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research. Neuron 2006;51:527–39.3.
3. Soares J, Marques P, Alves V, et al. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 2013;7:31.4.
4. Labate A, Cherubini A, Tripepi G, et al. White matter abnormalities differentiate severe from benign temporal lobe epilepsy. Epilepsia 2015;56:1109–16.5.
5. Jiang Y, Mao L, Yan X, et al. Investigation of altered microstructure in patients with drug refractory epilepsy using diffusion tensor imaging. Neuroradiology 2017;59:597–608.6.
6. Cox SR, Ritchie SJ, Tucker-Drob EM, et al. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nature Communications 2016;7:13629.7.
7. Anazodo UC, Shoemaker JK, Suskin N, et al. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. NeuroImage: Clinical 2013;3:388–95.8.
8. Anazodo UC, Shoemaker JK, Suskin N, et al. Impaired Cerebrovascular Function in Coronary Artery Disease Patients and Recovery Following Cardiac Rehabilitation. Front Aging Neurosci 2016;7:224.9.
9. Wiest-Daesslé N, Prima S, Coupé P, et al. Rician Noise Removal by Non-Local Means Filtering for Low Signal-to-Noise Ratio MRI: Applications to DT-MRI. In: Metaxas D, Axel L, Fichtinger G, et al., eds. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2008.Vol 5242. Berlin, Heidelberg: Springer Berlin Heidelberg; 2008:171–9.10.
10. Coupé P, Yger P, Prima S, et al. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging 2008;27:425–41.11.
11. Coupé P, Manjón JV, Gedamu E, et al. Robust Rician noise estimation for MR images. Medical Image Analysis 2010;14:483–93.12.
12. Kellner E, Dhital B, Kiselev VG, et al. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine 2016;76:1574–81.13.
13. Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 2019;202:116137.14.
14. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009;45:S173–86.15.
15. Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 2011;54:2033–44.16.
16. Leemans A, Jeurissen B, Sijbers J, et al. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc Intl Soc Mag Reson Med 2009;17:3537.
Figures