0381
Upright vs. Supine MRI: Effects of body position on craniocervical CSF flow1Department of Radiology, New York University Grossman School of Medicine, New York City, NY, United States, 2FONAR Corporation, Melville, NY, United States
Synopsis
CSF exchange between the spinal cord and the cranium increases in asymptomatic human subjects when body position is shifted from upright to supine. This appears to be caused by an increase in CSF flow during diastole, in the caudo-cranial direction, and systole, in cranio-caudal direction. Extrapolation of the results showed that within a 24 hour timescale, the more time spent in the supine position (asleep) correlated with more CSF exchanged between the spinal cord and the intracranial space. These alterations can therefore play a major role in brain waste clearance, and possibly many neurodegenerative diseases as well as age-related ailments.
Introduction
Cerebrospinal fluid (CSF) circulates between brain and spinal canal and its flow is driven by cardiovascular brain pulsation, influenced by body position.Few studies have reported links between exposure to microgravity and CSF volume properties changes (1-3), however, effects of gravity on CSF hydrodynamics via changes in body orientation have yet to be fully investigated. Over the past few years, there has been increasing interest in the effect of gravity on physiological aging and intriguing correlations between sleep and brain waste clearance by neural fluid movements(4). In this light, it is important to understand how postural changes alter CSF hydrodynamics. Advanced MRI techniques have furthered traditional studies of flow evaluation, allowing examination of pulsatile CSF hydrodynamic properties. Here, we investigate changes in CSF flow and volume at the C2 cervical spinal subarachnoid space when the body position of healthy subjects is changed from upright to supine using flow-sensitive MRI.Methods
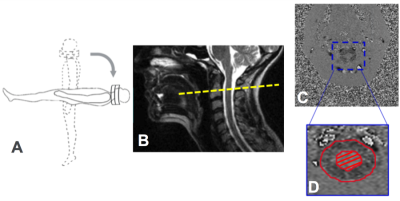
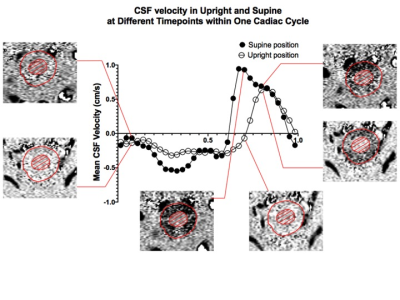
MRI scans from 30 asymptomatic volunteers (mean age=47±15; 9 males, 21 females) were studied retrospectively. For each subject, the MRI scans were first performed on a 0.6T MRI in the seated upright position, followed by acquisition in supine (Figure 1A). A quadrature cranio-cervical junction coil was used to image the foramen magnum and upper cervical spine region (Figure 1B). CSF flow and spinal cord pulsation were imaged with axial cine phase-contrast (PC) MRI using an RF-spoiled gradient echo sequence (TR=19 –22ms, TE=9–12ms, matrix=256x128 up-scaled to 256x256). Interleaved bipolar velocity encoding was used, resulting in an effective time resolution of two TR=38-44ms. 32 uniformly spaced time frames were obtained by linear interpolation in post-processing. In order to quantify the CSF flow, an axial slice of FOV=16cm at the mid-C2 cervical spinal level and perpendicular to the spinal canal was imaged (velocity encoding of about 5cm/s along the slice-select direction). From these PC images, the average pixel intensity over the CSF canal was extracted by manually drawing the ROIs (Figure 1C). This allowed for the calculation of CSF average velocity (Figure 2) and CSF volume flow, extracted from the area under the mean flow curve for diastole (negative caudo-cranial direction) and systole (positive cranio-caudal direction) separately. The CSF volume exchanged over one cardiac cycle, defined as stroke volume, was obtained by considering the smallest between the two areas. A two-tailed paired Student’s t-test was used to determine statistically significant differences in CSF hydrodynamic properties between the upright and supine positions.Results
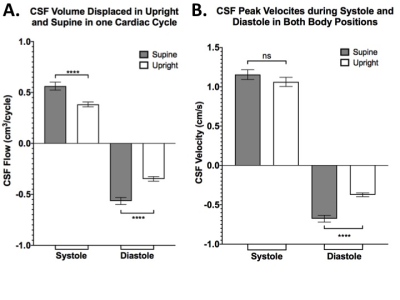
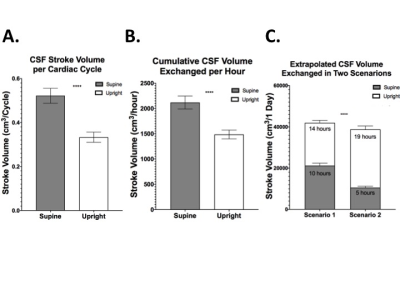
CSF properties were divided into systolic and diastolic components and then individually compared between the two body positions. Over one cardiac cycle, we observed that CSF flow and velocity have negative values (caudal-cranial direction) during diastole and positive values (cranio-caudal direction) in systole, in agreement with what reported in other studies (5). This subdivision led to the observation of a significant increase of CSF flow magnitude in the supine position, both systolic (0.56±0.04cm3/cycle) and diastolic (-0.57±0.03cm3/cycle), compared to upright: 0.38±0.13cm3/cycle and -0.35±0.12cm3/cycle, respectively (p<0.0001; Figure 3A). CSF peak velocity during diastole, guiding CSF into the cranium, significantly increased from -0.37±0.13cm/s in upright to -0.68±0.24cm/s in supine (p<0.0001). However, no significant changes were observed in systole, as evident from Figure 3B. One of our key findings is the clear distinction in CSF volume exchanged between the spinal and cranial space, measured as stroke volume. We showed that 69% more CSF was exchanged within one cardiac cycle in the supine position compared to upright (p<0.0001; Figure 4A). Two scenarios were then extrapolated from the CSF stroke volume per cardiac cycle results to facilitate large timescale clinical interpretation. Firstly, stroke volume exchanged over the course of one hour was extrapolated by adapting the one cardiac cycle data to the individual heart rate (HR) represented in figure 4B. Further projection of such results to the one day (24 hours) timescale (Figure 4C) showed a significantly higher exchange of CSF between spinal canal and cranium when 10 hours are spent in the supine position (scenario 1 for young people) as opposed to only 5 hours (scenario 2 for elderly; p<0.0001).Discussion
Recent studies have indicated that the pulsatile CSF movement is an important part of the glymphatic system for brain waste clearance, an area that has received growing attention. Our findings of 69% increased CSF flow at craniospinal junction level in supine versus upright position may provide important insights regarding neural fluid homeostatic (6), waste-clearance(7), and age-related neurodegeneration.Other studies also support a connection between CSF metabolic clearance and sleep (8), which led us to translate our findings from one cardiac cycle to a larger timescale for accumulative evaluation. Simulating time spent supine in both young and old subjects showed significantly higher CSF volume exchanged for longer times spent in supine.Conclusion
Our findings, demonstrate that body position has significant effects on the CSF hydrodynamic properties, measured at the cardiac cycle timescale as well as at simulated large timescale (e.g., 24 hours). Significantly reduced CSF exchange between the spinal cord and the cranium in the upright posture represents an innovative and promising finding for future studies to investigate the CSF-posture correlation in aging, as well as in a range of different neurological ailments.Acknowledgements
This study was funded by National Institute of Health grants (RF1 NS11041, R56 AG060822, R01 NS108491, R13 AG067684). This study is also supported by Alzheimer’s Association (AARG-17-533484).References
1. Van Ombergen A, Jillings S, Jeurissen B, Tomilovskaya E, Rumshiskaya A, Litvinova L, et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc Natl Acad Sci U S A. 2019;116(21):10531-6.
2. Kramer LA, Hasan, K.M., Sargsyan, A.E., Wolinsky, J.S., Hamilton, D.R., Riascos, R.F., Carson, W.K., Heimbigner, J., Patel, V.S., Romo, S., Otto, C. Mr‐derived cerebral spinal fluid hydrodynamics as a marker and a risk factor for intracranial hypertension in astronauts exposed to microgravity. Journal of Magnetic Resonance Imaging. 2015;42(6):1560-71.
3. Kramer LA, Hasan KM, Stenger MB, Sargsyan A, Laurie SS, Otto C, et al. Intracranial Effects of Microgravity: A Prospective Longitudinal MRI Study. Radiology. 2020;295(3):640-8.
4. Vernikos J, Schneider VS. Space, gravity and the physiology of aging: parallel or convergent disciplines? A mini-review. Gerontology. 2010;56(2):157-66.
5. Bunck AC, Kroger JR, Juttner A, Brentrup A, Fiedler B, Schaarschmidt F, et al. Magnetic resonance 4D flow characteristics of cerebrospinal fluid at the craniocervical junction and the cervical spinal canal. Eur Radiol. 2011;21(8):1788-96.
6. Spector R, Johanson C. Micronutrient and urate transport in choroid plexus and kidney: implications for drug therapy. Pharm Res. 2006;23(11):2515-24.
7. Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299-309.
8. Plog BA, Nedergaard, M. The glymphatic system in central nervous system health and disease: past, present, and future. . Annual Review of Pathology: Mechanisms of Disease. 2018;13:pp.379-94.
Figures