0379
Phase contrast MRI analysis of neurofluids in patients with Meniere’s disease and jugular venous stenosis.
Nivedita Agarwal1, Olivier Baledent2, Giuseppe Nicolò Frau3, and Sabino Walter Della Sala1
1Radiology, APSS Ospedale Santa Maria del Carmine, Rovereto, Italy, 2University of Amiens, Amiens, France, 3Otorhinolaryngology, APSS Ospedale Santa Maria del Carmine, Rovereto, Italy
1Radiology, APSS Ospedale Santa Maria del Carmine, Rovereto, Italy, 2University of Amiens, Amiens, France, 3Otorhinolaryngology, APSS Ospedale Santa Maria del Carmine, Rovereto, Italy
Synopsis
Meniere’s disease patients have a high incidence of abnormal neck venous vessels. An inefficient intracranial venous outflow would disturb global neurofluids dynamics and alter intracranial pressure. We used phase contrast MRI to correlate the dynamics of total arterial, total venous and CSF with morphological alterations in internal jugular veins (IJV). Our results show that although >80% of patients have IJV stenosis and isolated CSF and/or blood flow abnormalities, the global neurofluid dynamics remained unhampered. These results underscore the optimal compensation provided by collateral venous pathways and suggests that PC-MRI is an important adjunct clinical tool to to study neurofluids dynamics.
Background
Meniere’s disease (MD) is characterized by tinnitus, hearing loss and a sensation of aural fullness. Vascular mechanisms that affect an efficient drainage of venous flow from the intracranial compartment have been proposed as predisposing factors to the development of this disease (1,2). Stenosis of internal jugular veins (IJV) in MD has been reported in the literature proposing that symptoms of MD may be related to chronic cerebrospinal venous insufficiency (CCSVI) and some propose surgical intervention to improve IJV flow (3). However, such abnormalities have also been reported in other diseases such as multiple sclerosis and is highly prevalent in otherwise healthy subjects (4,5). Phase contrast MRI (PC-MRI) is an MR technique that allows quantification of neurofluid dynamics within the brain and spine. It can measure the total intracranial volume expansion in response to arterial inflow during each cardiac cycle (CC) providing insight on brain compliance and intracranial pressure (ICP) (6–8). We present a retrospective analysis of PC-MRI data in MD patients and correlate the findings to the presence of IJV stenosis and collateral venous flow.Methods
24 consecutive MD patients who had both PC-MRI and contrast enhanced MR venography (CE-MRV) were included. PC-MRI was acquired at the aqueduct of Sylvius (AoS) and at the C2-C3 spinal level (C2C3) to quantify arterial blood inflow to the brain (tA), venous outflow (tV) and CSF flow within the AoS (CSFaos) and C2C3 level (CSFc2c3). This data was analyzed using a validated inhouse software to calculate CSF stroke volume (SV), tA and tV flow. From these measurements, intracranial blood volume change during the CC, pulsatility (maximum and minium flow) of the tA and tV were calculated (6,9) (Figure 3). CE-MRV was acquired by injecting a standard dose of gadolinium intravenously. The morphology of the vessels was analyzed using Horos software. The cross-sectional area (CSA) of the IJV just below the IJV bulbs was calculated using manually drawn ROI and used as a reference to quantify stenosis. IJV was divided into two segments, upper (from C2-C4) and lower (C5-T1) for a total of 96 segments bilaterally (48 on each side). A reduction of >50% of IJV diameter with respect to JB diameter was identified and recorded as upper, lower, unilateral or bilateral stenosis. Condylar veins were evaluated the presence of efficient collateral venous outflow pathways since these veins represent the major link between intracranial dural venous sinuses and the vertebral venous plexi (10). All results were compared to data of age and sex-matched healthy subjects, already available in the literature (5,10).Results
15/9 (m/f) with an average age of 61.3 ±10.8y (range 39-77y) presented with a definite diagnosis of MD. Venous findings: right IJV was dominant in 62.5%, left IJV in 16.7% and was co-dominant in 20.8%. 21/24 patients (87.5%) had a stenosis in at least one segment. A stenosis was found in 44 of 96 segments (45.3%) of which 30 (68%) were in the upper segments of at least one of the two IJVs. Robust collateral veins were present in 21/24 patients (87.5%) not associated with the side of the stenosis (Figure 1,2). The mean degree of stenosis in the upper segments was 80.2 ± 11.7mm2 and in the lower segments was 77.92±16.35mm2 (p=0.29). PC-MRI findings (Figure 3): mean tA flow = 543±143 ml/min (two patients presented flow CAF >900); mean tV flow =324±155 ml/min. In 11 patients mean tV was 2.3±2 time smaller than tA inflow; CSFaos SV= 42 ±23 microL per CC (1 patient presented SV >80) and no correlation was found with the blood flow; CSFc2c3 SV=462 ±178 microL per CC (3 patients presented SV < 170 and 1>700); intracranial blood volume change during CC (890±640 microL) and was not well correlated with CSF c2c3 SV (R2=0.21), jugular pulsatility was not correlated with CSFc2c3, but CSF c2c3 was well correlated with arterial pulsatility amplitude (R2=0.63) (Figure 4, 5).Conclusion
This is the first study to correlate the dynamics of neurofluids in and out of the intracranial compartment in patients with definite diagnosis of MD with the presence of IJV stenosis and collateral neck vessels. In MD, IJV flow is highly decreased in comparison of arterial input flow, but paradoxically this important dynamic alteration does not significantly affect intracranial or spinal CSF flows.Acknowledgements
No acknowledgement found.References
1. Toro EF, Borgioli F, Zhang Q, Contarino C, Müller LO, Bruno A. Inner-ear circulation in humans is disrupted by extracranial venous outflow strictures: Implications for Ménière’s disease. Veins Lymphat [Internet]. 2018 Jan 8;7(1):1–12. 2. Gussen R. Vascular mechanisms in Meniere’s disease. Otolaryngol Head Neck Surg [Internet]. 1983 Feb 1;91(1):68–71. 3. Bruno A, Napolitano M, Califano L, Attanasio G, Giugliano V, Cavazzuti PP, et al. The Prevalence of Chronic Cerebrospinal Venous Insufficiency in Meniere Disease_ 24-Month Follow-up after Angioplasty. JVIR [Internet]. 2017 Mar 1;28(3):388–91. 4. Jayaraman M V., Boxerman JL, Davis LM, Haas RA, Rogg JM. Incidence of extrinsic compression of the internal jugular vein in unselected patients undergoing CT angiography. Am J Neuroradiol. 2012;33(7):1247–50. 5. Beggs CB, Magnano C, Shepherd SJ, Marr K, Valnarov V, Hojnacki D, et al. Aqueductal cerebrospinal fluid pulsatility in healthy individuals is affected by impaired cerebral venous outflow. J Magn Reson Imaging [Internet]. 2013 Nov 8;40(5):1215–22. 6. BALÉDENT O, HENRY-FEUGEAS M-CC, IDY-PERETTI I. Cerebrospinal Fluid Dynamics and Relation with Blood Flow. Invest Radiol. 2001;36(7):368–77. 7 Alperin NJ, Lee SH, Loth F, Raksin PB, Lichtor T. MR-intracranial pressure (ICP): A method to measure intracranial elastance and pressure noninvasively by means of MR imaging: Baboon and human study. Radiology. 2000. 8. Bateman GA. Vascular compliance in normal pressure hydrocephalus. AJNR Am J Neuroradiol [Internet]. 2000 Oct 1;21(9):1574–85. 9. Lokossou A, Metanbou S, Gondry-Jouet C, Baledent O. Extracranial versus intracranial hydro-hemodynamics during aging: a PC-MRI pilot cross-sectional study. Fluids Barriers CNS [Internet]. 2019 Dec 28;17(1):1–11. 10. San Millán Ruíz D, Gailloud P, Rüfenacht DA, Delavelle J, Henry F, Fasel JHD. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol [Internet]. 2002 Oct 1;23(9):1500–8. 11. Mortamet B, Zeng D, Gerig G, Prastawa M, Bullitt E. Effects of healthy aging measured by intracranial compartment volumes using a designed MR brain database. Med Image Comput Comput Assist Interv [Internet]. 2005 Jan 1;8(Pt 1):383–91.Figures
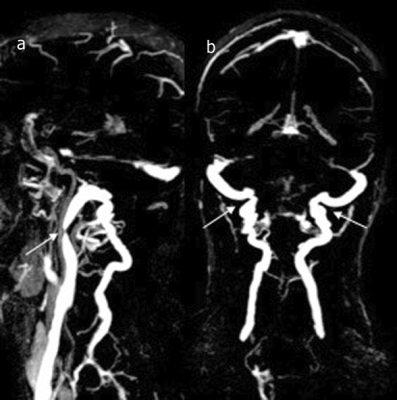
a) stenosis in the upper segment of the right IJV (white arrow). b) the presence of robust posterior and lateral condylar and deep cervical veins (double white arrows).
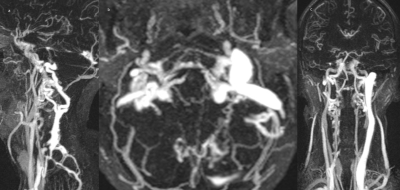
axial, coronal and sagittal CE-MRV- MIP images showing an atresic upper right IJV and robust collateral veins on both sides.
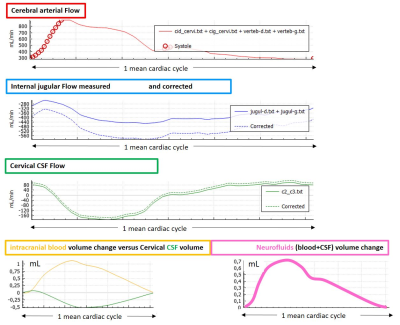
The curves represent overall neurofluid dynamics during cardiac cycle (CC). Cerebral arterial flow (red) (carotid and vertebral), IJV I(blue) was corrected (dotted line) to equal cerebral arterial flow. Intracranial blood volume change during CC (yellow), intracranial CSF volume change (green). Note how CSF responds to the intracranial blood pulsation. Nevertheless, CSF volume doesn’t fully compensate the blood volume and a net intracranial volume change is seen (pink).
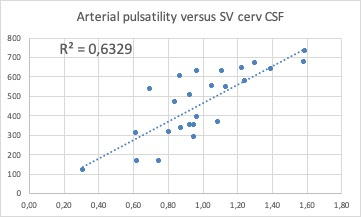
Direct correlation between the intracranial arterial pulsatility and CSFc2c3 stroke volume. This suggests that CSF outflow efficiently compensates arterial inflow
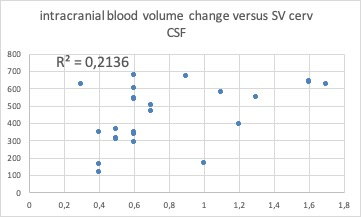
No correlation between intracranial blood volume changes during cardiac cycle with total stroke volume of CSF at the level of C2-C3 level. This suggests that the efficient collateral venous drainage allows for regular compensatory CSF flow at the level of C2C3.