0371
Ex Vivo Radiologic-Histologic Correlation of Pancreas Adenocarcinoma: A Feasibility Study1University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 2Georgetown University, Washington DC, MD, United States
Synopsis
The purpose of this study was to assess the feasibility of a novel radiologic-histologic correlation device RHCD and ex vivo MRI to facilitate direct correlation of radiologic and histologic features of pancreas cancer. Our approach is based on previous radiologic-histologic correlation of pancreatic anatomy using cadaveric pancreas specimens. The final protocol was applied to co-localize pancreas cancer margins radiologically and histologically, as well as nodal burden in pancreaticoduodenectomy specimens.
Introduction
Discrepancies between radiologic and pathologic tumor margins, as well as nodal burden in pancreas cancer are a major challenge that have important prognostic implications in the treatment of pancreas cancer1-4. Discrepancies between radiologic and histologic findings are secondary to the challenges of in vivo pancreas imaging and the complexity of pathologic processing5-7. Previous to this study, a protocol to correlate radiologic and histologic anatomy in human cadaveric pancreas specimens was created8. The protocol was then adapted for radiologic-histologic correlation of resected human pancreas adenocarcinoma specimens.The purpose of this work was to assess the feasibility of application of the adapted protocol to co-localize radiologic and histologic tumor margins and tumor features using ex vivo MRI and a novel radiologic-histologic correlation device (RHCD) in resected human pancreas cancer specimens.
Methods
SpecimensInstitutional review board (IRB) approval and informed consent was obtained for this prospective study. Pancreaticoduodenectomy specimens were obtained from patients undergoing curative resection for pancreas adenocarcinoma. Per existing institutional pathology protocol, the specimens were re-inked, sectioned longitudinally, and fixed in formalin for 24 hours prior to any further processing or imaging.
Radiologic-histologic correlation device (RHCD)
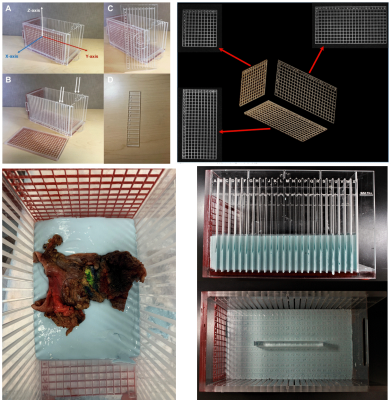
Briefly, the RHCD is a Plexiglas 27x14x14cm3 MRI-compatible device with 1cm grid patterns in 3 dimensions, visible on MR through silicone gel infilling, and sectioning planes in the x-axis orientation (Figure 1). The RHCD has been previously validated within the context of radiologic-histologic correlation of liver tumors 9 and cadaveric pancreas specimens 8. These studies demonstrated excellent radiologic-histologic correlation of tumor location and anatomy.
After formalin fixation, specimens were placed in a casting material (alginate) within the RHCD (Figure 1) to maintain an axial orientation relative to the sectioning axis.
MR Protocol
All specimens were then imaged using a clinical 3T MRI system (GE Signa PET/MR, GE Healthcare, Waukesha WI) using a high-resolution (0.8mm isotropic) fat suppressed 3D T1-weighted fast spoiled gradient echo (T1w-SGRE) and a 3D fat suppressed T2-weighted fast-spin-echo (T2w-FSE). MR images were acquired using a single channel quadrature head coil. Acquisition parameters for T1w 3D-SGRE scan included: TR=12.3ms, TE=1.7ms, RBW=+/-62.5kHz, FOV=14.4x22.4x32cm, Matrix size=180x280x416, spatial resolution=0.8x0.8x0.8mm3, flip angle=20 , and scan time=10:19. Acquisition parameters for T2w 3D-FSE included: TR=3.3s, TE=100ms, ETL=130, RBW=+/-62.5kHz, FOV=14.4x22.4x32cm, Matrix size=180x280x416, spatial resolution=0.8x0.8x0.8mm3 and scan time=16:10.
MRI Interpretation
Following imaging, study radiologists reviewed all images and recorded the RHCD coordinate location of the tumor and any additional points of interest, such as anticipated positive margins or suspicious lymph nodes.
Pathologic Processing and Analysis
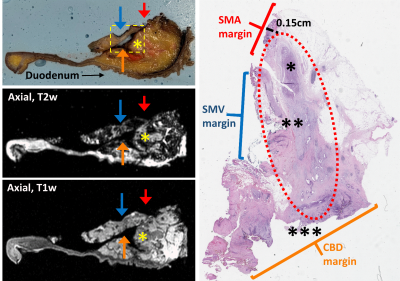
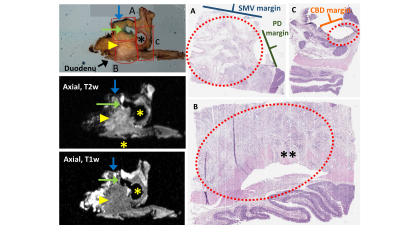
After imaging, specimens were sectioned axially via the x-axis sectioning planes. Specimens were then processed according to standard of care pathologic protocols currently in use for surgical specimens within the Department of Pathology. Tissue-containing cassettes were prepared for each specimen and captured tumor in relation to all pertinent margins and anatomical landmarks, including the pancreas neck, superior mesenteric vein (SMV), superior mesenteric artery (SMA), common bile duct (CBD), pancreatic duct (PD), and ampulla of Vater. Each cassette was labeled per standard protocol and RHCD coordinates were recorded in the gross description corresponding to the sub-section of specimen. All identifiable peripancreatic and perigastric nodes were placed in cassettes in a similar manner. Cassettes then underwent tissue processing (dehydration, clearing, and paraffin embedding) and were sectioned for histologic examination.
Radiologic-Histologic Correlation
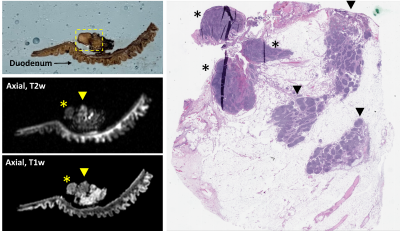
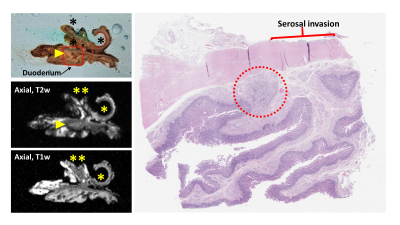
Using the RHCD coordinates, histologic findings were compared to the co-localized MRI findings. This required coordination and collaboration between the study pathology and radiology teams. Each histology slide that contained tumor was scanned and referenced by the radiology team during their re-review of the attained images. Using this histologic reference, study radiologists described the corresponding MRI findings, as co-localized via the RHCD coordinates.
Results
To date, 11 human pancreas cancer specimens have been processed with the described protocol. All 11 specimens were successfully processed using the protocol; there were no adverse events and all patients had appropriate pathologic staging. There was excellent (100%) correlation between radiologist-perceived and histology-confirmed tumor size and location. In review of the prepared histology slides, radiologists consistently found the corresponding specimen tissue and defined its anatomy and orientation in relation to adjacent margins. Examples of radiologic-histologic correlation of 2 pancreas specimens are presented in Figures 2-5.Discussion
Radiologic histologic correlation using ex vivo MRI in pancreas specimens is feasible. This approach conveys precise anatomic relationships between the pancreas and adjacent structures that are not visible with in vivo imaging. This protocol and technology can be used to better assess radiologic markers of tumor margin, nodal status, and treatment response to systemic and local therapies.Conclusion
It is feasible to use ex vivo MRI and this novel RHCD to co-localize radiologic and histologic tumor components, margins, and nodal burden in resected pancreas adenocarcinoma specimens. This protocol and technology should be evaluated further in future studies aimed at better characterizing radiologic features of pancreas adenocarcinoma.Acknowledgements
We wish to acknowledge support from the University of Wisconsin Carbone Cancer Center's Pancreas Pilot Grant and the University of Wisconsin Institute for Clinical and Translational Research. Further, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Porembka MR, Hawkins WG, Linehan DC, et al. Radiologic and intraoperative detection of need for mesenteric vein resection in patients with adenocarcinoma of the head of the pancreas. HPB (Oxford). 2011;13(9):633-642.
2. Kluger MD, Rashid MF, Rosario VL, et al. Resection of Locally Advanced Pancreatic Cancer without Regression of Arterial Encasement After Modern-Era Neoadjuvant Therapy. J Gastrointest Surg. 2018;22(2):235-241.
3. Ghaneh P, Kleeff J, Halloran CM, et al. The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269(3):520-529.
4. Strobel O, Hank T, Hinz U, et al. Pancreatic Cancer Surgery: The New R-status Counts. Ann Surg. 2017;265(3):565-573.
5. Verbeke CS. Resection margins in pancreatic cancer. Pathologe. 2013;34 Suppl 2:241-247.
6. Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic head cancer. Br J Surg. 2012;99(8):1036-1049.
7. Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford). 2009;11(4):282-289.
8. Acher AW CT, Zhang Y, Kovacs K, Starekova J, Rendell V, Abbott DE, Brooks E, Agni R, Winslow E, Reeder DB. Ex Vivo MRI for Direct Radiologic-Histologic Correlation in the Pancreas: Protocol Development with Cadaver Specimens. Paper presented at: ISMRM 20202020; Virtual.
9. Rendell V, Timothy J. Colgan, Gesine Knobloch, Matthias R. Muhler, Agnes G Loeffler, Rashmi M. Agni, Krisztian Kovacs, Emily R. Winslow, Scott B. Reeder. Direct Radiologic-Pathologic Correlation of Liver Lesions with an MR-Compativle Sectioning and Localization Device. In. ISMRM Annual Meeting and Exhibition. 2019.
Figures