0370
Assessment of pancreatic tumour response on LDE225, gemcitabine and nab-paclitaxel using Intravoxel Incoherent Motion MRI1Department of Radiology and Nuclear Medicine, Cancer Center Amsterdam, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands, 2Department of Medical Oncology, Cancer Center Amsterdam, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
Synopsis
Stromal deposition can become a physical and biological barrier that prevents chemotherapy from reaching pancreatic ductal adenocarcinoma (PDAC). In this study, 34 patients were treated with LDE225, which specifically targets tumour stroma, gemcitabine and nab-paclitaxel. Pancreatic tumour response was assessed using intravoxel incoherent motion (IVIM) MRI. We found that the diffusion in PDAC increased after chemotherapy, which may be explained by reduction of stroma or tumour necrosis. Furthermore, a positive correlation was found between overall survival and the change in tumour perfusion, underlining the fact that reperfusion of PDAC by LDE225 improves prognosis.
Introduction
For patients with metastatic PDAC palliative chemotherapy is the standard of care[1-2]. But despite the progress made in treatment of advanced PDAC over the past years, survival remains poor. Stromal deposition is thought to play a crucial role in preventing chemotherapy efficacy, being a physical and biological barrier that limits the vascularization of the tumour[2-5]. Hence, chemotherapy efficacy may be improved by targeting the stroma alongside the tumour. Therefore, we designed a study in which we treated PDAC patients with LDE225 combined with conventional chemotherapy of gemcitabine and nab-paclitaxel. LDE225 is thought to specifically target the tumour’s stroma[5-6]. By imaging tumour microenvironment, we may get a better insight in how this novel treatment affects PDAC in this patient population.A promising microenvironment imaging approach is IVIM MRI[7]. IVIM provides information on tissue diffusion and perfusion, two very promising biomarkers in the context of stroma and treatment response[8]. Stroma contains dense tissue, resulting in restricted diffusion and limiting vascularity, leading to poor perfusion. The goal of this research is to assess PDAC’s response to LDE225 and gemcitabine+nab-paclitaxel with IVIM MRI and to correlate IVIM parameters with the overall survival (OS) of these patients.
Method
34 patients with metastatic PDAC were included in this study. As part of the study, they received treatment with LDE225 and gemcitabine+nab-paclitaxel. As we combined the data from the phase 1 and phase 2 part of the trial, the dose of LDE225 differed per patient from 200mg(n=18), 400mg(n=8), 600mg(n=3) to 800mg(n=4). All patients underwent a baseline MRI scan before the start of the chemotherapy and 21 patients also underwent a second MRI scan after 8 weeks of treatment. Furthermore, the OS of 28 patients was recorded of whom 18 underwent both MRI scans.All MRI examinations were conducted using a 3T MRI scanner (Ingenia, Philips, Best, Netherlands). Diffusion Weighted Imaging (DWI) was performed using single shot 2D echo planar imaging with TR/TE:2145/46ms, SENSE factor:1.3, SPIR and gradient reversal for fat suppression, b-values(directions/averages):0(15),10(9),20(9),30(9),40(9),50(9),75(4),100(12),150(4),250(4),400(4),600(16). Respiratory triggering with a diaphragm navigator was used.
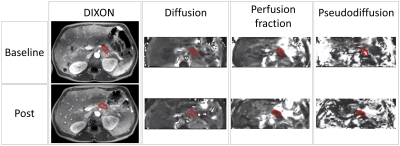
Per voxel, the IVIM model was fitted to the signal decay as a function of the b-value using a bi-exponential fit to obtain diffusion (D), pseudodiffusion (D*) and perfusion fraction (f) maps. The pancreatic tumour was manually delineated on baseline and post-treatment MRI scans under guidance of a post-contrast MRI from the same scan session (Figure 1). The mean parameter values from within the ROIs were used for further analysis.
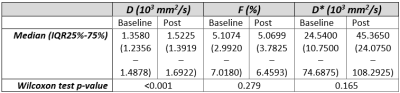
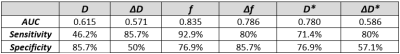
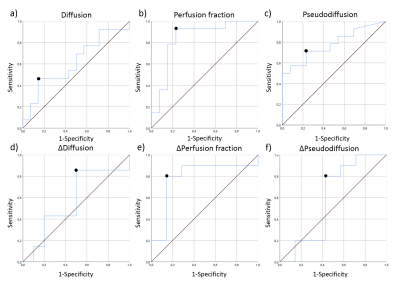
All statistical tests were two-tailed and a significance level of 0.05 was used. Data were first tested on normality using the Shapiro-Wilk test. The overall effect of the chemotherapy on the tumour was assessed by a Wilcoxon signed-rank test between MRI scans at baseline and post-chemotherapy for all IVIM parameters. To test whether IVIM parameters correlated to OS, a Spearman’s Rank Correlation test was done. Subsequently, a Receiver Operating Characteristic (ROC) analysis was performed to determine the specificity and sensitivity (using the Youden’s index) of baseline IVIM parameters and the relative change in parameter value during treatment to predicting OS of PDAC patients receiving chemotherapy. OS of this cohort was between 35 days and 1,5 years. The mean OS, 230 days, was taken as cut-off value to determine survivors for the purpose of the ROC analysis.
Results
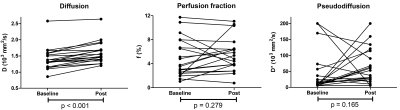
The Wilcoxon test showed that only D significantly increased during chemotherapy (Figure 2+3). Furthermore, significant correlations were found between the OS and baseline f (rs=-0.46,p=0.016) and between OS and the relative difference of f (Δf) between baseline and post chemotherapy (rs=0.50,p=0.040). The AUC, sensitivity and specificity of each parameter can be seen in Figure 4. Noticeably, f (Figure 5b) showed high AUC values. No relation was found between OS and dose of LDE225.Discussion
We showed that IVIM is able to detect treatment effects from combined LDE225, gemcitabine and nab-paclitaxel in PDAC patients. We showed that diffusion significantly increased during treatment. Furthermore, we showed that an increase in perfusion fraction during treatment resulted in a better prognosis. We believe that two mechanisms contributed to this increase, namely necrosis and the decrease in stroma. The accompanying lower cellularity causes a higher diffusion[9].Whether or not the increase in D found in this population, and accompanying reduction in stroma, also increased tumour perfusion, turned out to be patient-specific (i.e. figure 3). In fact, on average, the perfusion decreased slightly. Potentially, there is an interplay between perfusion increasing factors, such as LDE255-induced reduction in stroma, and perfusion decreasing factors, such as the effect of regular chemotherapy. A positive correlation between the Δf and the OS was found, where patients that had larger increase in f had better prognosis. We believe this highlights that when the LDE225-induced stroma reduction indeed leads to more perfusion, patients had better OS. Our findings highlight the importance of measuring IVIM during LDE255 chemotherapy to predict treatment efficacy.
Conclusion
This study shows that after treatment of PDAC with the combination of LDE225 and gemcitabine+nab-paclitaxel, the diffusion of the tumour increased based on IVIM parameters. Furthermore, there seems to be a positive correlation between OS and the change in f due to chemotherapy.Acknowledgements
No acknowledgement found.References
[1] Kleeff, J., Korc, M., Apte, M., La Vecchia, C., Johnson, C. D., Biankin, A. V., Neale, R. E., Tempero, M., Tuveson, D. A., Hruban, R. H., & Neoptolemos, J. P. (2016). Pancreatic cancer. Nature Reviews Disease Primers, 2(April), 1–23. https://doi.org/10.1038/nrdp.2016.22
[2] Bijlsma, M. F., & van Laarhoven, H. W. M. (2015). The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: a systematic review and critical appraisal. Cancer and Metastasis Reviews, 34(1), 97–114. https://doi.org/10.1007/s10555-014-9541-1
[3] Xu, Z., Pothula, S. P., Wilson, J. S., & Apte, M. V. (2014). Pancreatic cancer and its stroma: A conspiracy theory. World Journal of Gastroenterology, 20(32), 11216–11229. https://doi.org/10.3748/wjg.v20.i32.11216
[4] Feig, C., Gopinathan, A., Neesse, A., Chan, D. S., Cook, N., & Tuveson, D. a. (2013). The pancreas cancer microenvironment. Clinical Cancer Research, 18(16), 4266–4276. https://doi.org/10.1158/1078-0432.CCR-11-3114
[5] Erkan, M., Reiser-Erkan, C., Michalski, C. W., Kong, B., Esposito, I., Friess, H., & Kleeff, J. (2012). The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Current Molecular Medicine, 12(3), 288–303. https://doi.org/10.2174/156652412799218921
[6] Zhou, J., Quinlan, M., Hurh, E., & Sellami, D. (2016). Exposure-Response Analysis of Sonidegib (LDE225), an Oral Inhibitor of the Hedgehog Signaling Pathway, for Effectiveness and Safety in Patients With Advanced Solid Tumors. Journal of Clinical Pharmacology, April, 1406–1415. https://doi.org/10.1002/jcph.749
[7] Le Bihan, D., Breton, E., Lallemand, D., Aubin, M. L., Vignaud, J., & Laval-Jeantet, M. (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 168(2), 497–505. https://doi.org/10.1148/radiology.168.2.3393671
[8] Klaassen, R., Steins, A., Gurney-Champion, O. J., Bijlsma, M. F., van Tienhoven, G., Engelbrecht, M. R. W., … van Laarhoven, H. W. M. (2020). Pathological validation and prognostic potential of quantitative MRI in the characterization of pancreas cancer: preliminary experience. Molecular Oncology, 14, 2176–2189. https://doi.org/10.1002/1878-0261.12688
[9] Heid, I., Steiger, K., Trajkovic-Arsic, M., Settles, M., Eßwein, M. R., Erkan, M., … Braren, R. F. (2017). Co-clinical assessment of tumor cellularity in pancreatic cancer. Clinical Cancer Research, 23(6), 1461–1470. https://doi.org/10.1158/1078-0432.CCR-15-2432
Figures