0368
Hyperpolarized 13C Metabolic Imaging of Patients with Pancreatic Ductal Adenocarcinoma1Department of Radiology & Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Department of Medicine, University of California, San Francisco, San Francisco, CA, United States, 3UC Berkeley-UCSF Graduate Program in Bioengineering, San Francisco, CA, United States
Synopsis
Metabolic imaging of hyperpolarized [1-13C]pyruvate was performed in four patients with pancreatic ductal adenocarcinoma prior to chemotherapy. Compared to the normal appearing pancreas, higher lactate/pyruvate and lower alanine/pyruvate ratios were seen in the primary tumors. The alanine/lactate ratio, which reflects the relative enzymatic activity and metabolite pool sizes, was reduced 2- to 20-fold in the tumors and provided improved contrast between tumor and normal appearing pancreas over either the lactate/pyruvate or alanine/pyruvate ratio. These results indicate the potential for HP 13C MRI to be a novel tool to stage disease and assess treatment response in pancreatic cancer patients
Introduction
Pancreatic ductal adenocarcinoma (PDA) is the 3rd leading cause of cancer related death in the US and is anticipated to become the 2nd by 2030 (1). Most PDA patients present with advanced disease for which systemic chemotherapy is the only current life-prolonging treatment. However, assessing early response to treatment, particularly of the primary tumor, is challenging since changes in tumor size occur late, and viable tumor cannot be reliably differentiated from fibrosis (2). As a result, many patients are discovered to have progressive disease after months of ineffective systemic therapy when they are no longer suitable for second-line therapy or clinical trials. Therefore, there is an urgent unmet need for more accurate imaging methods to assess early therapeutic response/resistance, which will minimize toxicities of ineffective treatments and enable alternative treatments with potentially better efficacy.Hyperpolarized (HP) 13C MRI is an emerging technology that overcomes the poor sensitivity of 13C nuclei, enabling dynamic imaging of both the injected substrate and downstream metabolites. Given the demonstrated glycolytic metabolic reprogramming that occurs during PDA tumorigenesis (3,4), HP 13C pyruvate MRI has the potential to enable staging/restaging and assess treatment response in a non-invasive, radiation-free manner in patients with PDA. In this work, we investigate the feasibility of imaging PDA metabolism using HP 13C pyruvate and demonstrate that it is a robust and novel platform to study PDA metabolism, with the potential to characterize the presence and extent of aggressive pancreatic cancer and its response to therapy.
Methods
Patient studies (n=4) were performed on a 3T MR scanner (MR750, GE Healthcare) equipped with clinical performance gradients (Gmax = 50mT/m, Smax = 200T/m/s) following FDA IND and IRB-approved protocols. All four patients were imaged prior to the start of a line of therapy (Table 1). Anatomic images were acquired with the built-in body coil and consisted of a multi-slice SSFSE, 3D SPGR (LAVA, pre- and post-contrast), multi-echo IDEAL, and DWI. For the HP 13C study, an aliquot of 1.47g of Good Manufacturing Practice (GMP) grade [1-13C]pyruvic acid (MilliporeSigma Isotec) and 15mM trityl electron paramagnetic agent (AH111501, GE Healthcare) was prepared by a pharmacist just prior to the study and polarized using a SPINlab polarizer (GE Healthcare). Following dissolution and release by the pharmacist, a 0.43mL/kg dose of pyruvate was injected at a rate of 5mL/s, followed by a 20mL sterile saline flush.HP 13C data were acquired with either singe-slice EPSI (n=1) or a multi-slice metabolite-selective EPI sequence (5) (n=3) using a flexible vest coil for RF transmit and an 8-channel array for receive (QTAR, Clinical MR Solutions), with an in-plane resolution of 2x2cm2 and (for the EPI) seven 2.0cm slices. Twenty timeframes were acquired with a 3s effective temporal resolution, yielding a total scan time of one minute. Multichannel HP 13C EPI data were reconstructed as described previously (6). For analysis, SNR and area under the curve (AUC) ratios were measured from ROIs over the primary tumor and normal appearing pancreas.
Results & Discussion
Injections of HP [1-13C]pyruvate were well tolerated by all patients, and no adverse events were reported. The mean polarization was 37.9±3.6% and the injected pyruvate concentration was 246.2±19.1mM. The mean AUC SNR for pyruvate, lactate, and alanine was 100.4±42.7, 22.7±14.5, and 11.8±2.9 in the normal appearing pancreas, and 62.1±42.2, 21.6±5.9, and 3.9±5.0 in the tumor.Representative results from two patients (#3 and #4) can be seen in Figs. 1 and 2, and data from all four patients are summarized in Table 2. The metabolite AUCs (Figs. 1 and 2, top rows) reflect pyruvate perfusion and metabolism, along with the non-uniform receive profile for the 8-channel array. Normalizing to pyruvate removes the inhomogeneous transmit/receive profile (Figs. 1 and 2, bottom rows) and provides a measure of metabolism that is proportional to the forward rate constant (7). Higher lactate/pyruvate, which reflects lactate dehydrogenase (LDH) activity and lactate pool size, was seen in the primary tumor. Conversely, higher alanine/pyruvate, which reflects alanine transaminase (ALT) activity and alanine pool size, was seen in the normal appearing pancreas. The alanine/lactate ratio, which provides a relative measure of metabolism, was reduced 2- to 20-fold in the tumor and provides improved contrast over either the lactate/pyruvate or alanine/pyruvate ratios.
These results are consistent with pre-clinical studies showing a lower alanine/pyruvate and higher lactate/pyruvate with PDA tumorigenesis (4). Our finding of lower alanine/lactate in PDA compared to normal pancreas is opposite to that reported in a recent paper of HP [1-13C]pyruvate MRI in two PDA patients (8), and may be related to the long course of combination chemotherapy that those two patients had already received and responded to at the time of imaging. Our study is ongoing and will examine early metabolic changes following therapy using HP 13C pyruvate MRI.
Conclusion
This study demonstrates the safety and feasibility of HP 13C MRI in patients with PDA. In this study, a lower alanine/pyruvate and higher lactate/pyruvate ratio was observed in the primary tumor. Ongoing studies will explore the application of HP 13C pyruvate metabolic imaging as an innovative tool to stage and assess early treatment response in PDA.Acknowledgements
This work was supported by NIH grants P41EB013598 and a UCSF Resource Allocation Program Grant. We would also like to acknowledge Mary Frost, Kimberly Okamoto, and Namasvi Jariwala for their assistance with the patient studies, and to Andrew Riselli and Evelyn Escobar for preparing the fluid paths.References
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research 2014;74(11):2913-2921.
2. Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, Wang H, Abbruzzese J, Pisters PW, Vauthey JN, Charnsangavej C, Tamm E, Crane CH, Balachandran A. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118(23):5749-5756.
3. Vaziri-Gohar A, Zarei M, Brody JR, Winter JM. Metabolic Dependencies in Pancreatic Cancer. Front Oncol 2018;8:617.
4. Serrao EM, Kettunen MI, Rodrigues TB, Dzien P, Wright AJ, Gopinathan A, Gallagher FA, Lewis DY, Frese KK, Almeida J, Howat WJ, Tuveson DA, Brindle KM. MRI with hyperpolarised [1-13C]pyruvate detects advanced pancreatic preneoplasia prior to invasive disease in a mouse model. Gut 2016;65(3):465-475.
5. Gordon JW, Chen H-Y, Autry A, Park I, Van Criekinge M, Mammoli D, Milshteyn E, Bok R, Xu D, Li Y, Aggarwal R, Chang S, Slater JB, Ferrone M, Nelson S, Kurhanewicz J, Larson PEZ, Vigneron DB. Translation of Carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magnetic Resonance in Medicine 2019;81:2702– 2709.
6. Crane JC, Gordon JW, Chen H-Y, Autry AW, Li Y, Olson MP, Kurhanewicz J, Vigneron DB, Larson PEZ, Xu D. Hyperpolarized 13C MRI data acquisition and analysis in prostate and brain at University of California, San Francisco. NMR in Biomedicine 2020:e4280.
7. Hill DK, Orton MR, Mariotti E, Boult JKR, Panek R, Jafar M, Parkes HG, Jamin Y, Miniotis MF, Al-Saffar NMS, Beloueche-Babari M, Robinson SP, Leach MO, Chung Y-L, Eykyn TR. Model Free Approach to Kinetic Analysis of Real-Time Hyperpolarized 13C Magnetic Resonance Spectroscopy Data. PLoS ONE 2013;8(9):e71996.
8. Stødkilde-Jørgensen H, Laustsen C, Hansen ESS, Schulte R, Ardenkjaer-Larsen JH, Comment A, Frøkiær J, Ringgaard S, Bertelsen LB, Ladekarl M, Weber B. Pilot Study Experiences With Hyperpolarized [1-13C]pyruvate MRI in Pancreatic Cancer Patients. Journal of Magnetic Resonance Imaging 2019;0(0).
Figures
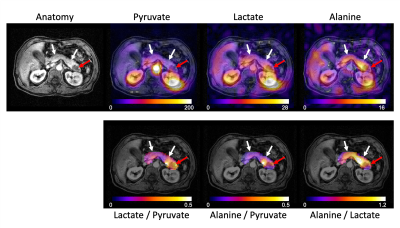
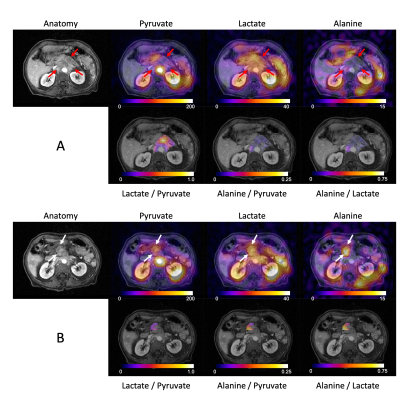
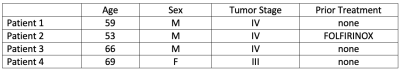
Table 1. Pancreatic ductal adenocarcinoma patient demographics. All four patients were imaged prior to the start of a line of therapy; three patients were newly diagnosed and treatment naive, one patient was a non-responder to a frontline therapy and was scheduled to start second line therapy.
FOLFIRINOX: combination chemotherapy of folinic acid, fluorouracil, irinotecan, and oxaliplatin.
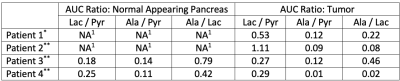
Table 2. Area under the curve (AUC) metabolite ratios for ROIs over the normal appearing pancreas and tumor.
1Metabolite ratios for normal appearing pancreas were not reported for Patients 1 and 2 because normal pancreas was not present in the single slice acquired using EPSI (patient 1), and tumor involved the entire pancreas (patient 2).
*C13 data of Patient 1 was acquired with EPSI.
**C13 data of Patients 2-4 were acquired with EPI.