0356
Transcriptomic decoding of the human brain structural connectome through the 3rd trimester and early childhood1Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, PA, United States, 3Neurogenomics group, Research Programme on Biomedical Informatics (GRIB), Hospital del Mar Medical Research Institute (IMIM), DCEXS, Universitat Pompeu Fabra, Barcelona, Spain, 4Department of Neuroscience and Kavli Institute for Neuroscience, Yale School of Medicine, New Haven, CT, United States, 5Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Transcriptome, the set of gene expression, is spatiotemporally heterogeneous across brain development. Under its regulation, dramatic changes in brain connectivity estimated through diffusion MRI is observed in early childhood. However, the association between the macroscopic structural connectome and microscopic transcriptome across early postnatal years is not clear. Here, we revealed this dynamic association between structural connectome and gene expression from a large cohort of 200 neonates and children through the 3rd trimester and early childhood. The changes of associated genes’ enrichment in cell types and biological processes across different ages shed light into the dynamic transcriptomic roles in connectome development.
Purpose
Through the 3rd trimester and early childhood, dramatic development in brain connectivity including synaptogenesis and myelination occurs in the brain [1-5]. Gene expression, which is dynamic across development and heterogeneously across brain regions, regulates this dramatic change at molecular and cellular level. Our previous work has found correlation between heterogeneous transcription level and cortical microstructure in fetus [6], however, there is still a gap on associations between macroscopic connectome and microscopic transcriptome (the set of gene expression) in early development [7]. In addition, whether and how the association changes across age are not well understood yet. In this study, we aimed to bridge this gap and to reveal the dynamic association between connectome and transcriptome in human early development from early 3rd trimester to 8 years after birth.Methods
Schematic pipeline of methods is shown in Figure 1.Subjects and MRI data acquisition: Two datasets, total 200 subjects spanning from the 3rd trimester to 8 years of age were included in this study. Both datasets were scanned with Phillips 3T Achieva MR scanners. Diffusion MRI (dMRI) were acquired using a single-shot EPI sequence: 30 independent diffusion-weighted directions, b-value=1000 s/mm2, repetition=2. For neonate dataset, high-resolution dMRI were acquired from 87 neonates (56M/31F) aged 29-40 postconceptional weeks (pcw), TR/TE=6850/78ms, FOV=168x168mm2, imaging resolution=1.5x1.5x1.6mm3, 60 slices. For early childhood dataset, 113 children (49M/64F) aged 0.17–7.91 years were scanned, TR/TE=9300/100ms, FOV=256x256mm2, imaging resolution=2x2x2mm3, 70 slices.
Connectome construction and structural network analysis: Whole-brain, deterministic tractography was performed in DiffusionToolkit (http://www.trackvis.org/dtk/), with FA threshold of 0.1 and angle threshold of 35 degrees. MNI single-subject template and dilated 256 uniform parcels (nodes) [8] of its FreeSurfer atlas were registered to individual b0 images. When building the weighted structural network, effects of resolution and number of voxels differences were taken into account, and network elements were calculated by [2]:
$$w_{i,j}=\frac{FN_{i,j} \times FA \times VoxelSize}{VN_i + VN_j}$$
Where $$$FN_{i,j}$$$=the number of fibers connecting node $$$i$$$ and $$$j$$$; $$$VN_i$$$=the number of voxels in node $$$i$$$. Degree centrality of each node was calculated based on graph theory, and its developmental trajectory were fitted with LOESS (locally estimated scatterplot smoothing).
mRNA profiling: Tissue-level mRNA sequencing was performed from 39 high-quality brain samples (21M/18F) aged 12pcw to 40 years from PsychENCODE [9]. Normalized mRNA level, RPKM (Reads Per Kilobase of transcript per Million mapped reads) of 60155 genes were measured from 11 neocortical regions. RPKM levels across ages were fitted with LOESS. Expression from genes that were differentially expressed (n=12640) were used. In addition, single-nuclei RNA sequencing (snRNA-seq) data was also generated from three adults.
Association between connectome and transcriptome, and gene enrichment analysis: To explore the association across development, multivariate dimension-reducing technique of partial least squares (PLS) regression was applied on fitted degree centrality and age-matched fitted RPKM at 35 age points from 30pcw to 8 years. At each age point, genes were ranked according to weights on PLS 1 component. The gene ontology enrichment analysis tools [10,11] were used to identify biological processes that were overrepresented in the associated genes. Enrichment in cell types was analyzed via Kolmogorov-Smirnov test with snRNA-seq dataset.
Results
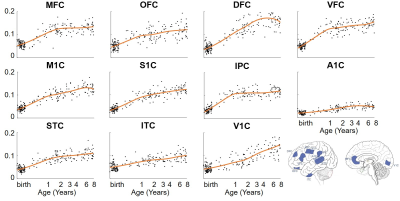
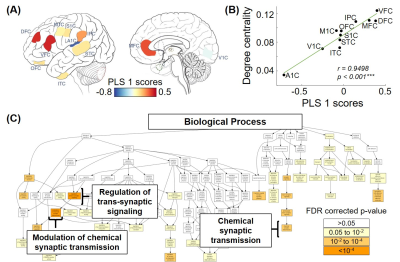
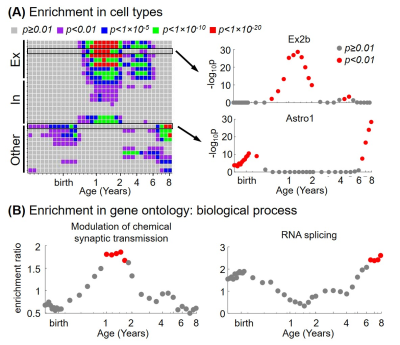
From the 3rd trimester to 8 years of age, degree centrality showed heterogeneous increase across brain regions, where prefrontal cortex and primary visual cortex developed faster and temporal cortex increased slower (Figure 2). Significant association between degree centrality and gene expression were found and Figure 3 showed an example at 1 year of age. 90.20% of variance in degree centrality was explained by the first PLS component (p=0.038, permutation test). The profile of genes identified by this first PLS component showed overexpression in prefrontal cortex (Figure 3A), and significant positive correlation with degree centrality (Figure 3B, r=0.9498, p<0.001). These associated genes were significantly enriched in gene ontology terms related to synaptic signaling and transmission and their regulations (Figure 3C, FDR p<0.01). In addition, we revealed dynamic enrichment in cell types (Figure 4A) and biological processes (Figure 4B) from the 3rd trimester to 8 years of age. For example, enrichment in Ex2b, a subtype of excitatory neuron, peaked at around 1.5 years of age, whereas enrichment in Astro1, a subtype of astrocyte, demonstrated an early peak at around birth and an increasing trend after 6 years (Figure 4A). For biological processes (Figure 4B), enrichment ratio in modulation of chemical synaptic transmission peaked at 1-2 years of age and RNA splicing peaked at around 7 years of age.Discussion and Conclusions
We revealed the dynamic association between connectome and gene expression through the 3rd trimester and early childhood. Age of 1-2 years is one of the highlights with gene enrichment peaks of excitatory and inhibitory neurons, and enrichment of modulation of chemical synaptic transmission. These peaks mirror the higher rate of synaptogenesis development in the first two years of life [1,12]. More interestingly, the differential peak ages in various cell types and biological processes may suggest their different roles across connectome development stages. Taken together, we identified gene sets, biological processes, and cell types important for connectome development at different ages during early childhood. Future studies may focus on the vulnerability of these highlights in neurodevelopmental diseases.Acknowledgements
This study is funded by NIH MH092535, MH092535-S1 and HD086984. The transcriptome data was generated with the support of The BrainSpan Project Consortium by grants MH089929, MH090047, and MH089921 from NIMH. In addition, G.S. received the support of a fellowship from “la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/PI19/11690010. G.S. is also supported by Ministerio de Ciencia e Innovación, Spain (PID2019-104700GA-I00).References
[1] Ouyang, M., Dubois, J., Yu, Q., Mukherjee, P., & Huang, H. (2019). Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage, 185, 836-850.
[2] Song, L., Mishra, V., Ouyang, M., Peng, Q., Slinger, M., Liu, S., & Huang, H. (2017). Human fetal brain connectome: structural network development from middle fetal stage to birth. Frontiers in neuroscience, 11, 561.
[3] Zhao, T., Mishra, V., Jeon, T., Ouyang, M., Peng, Q., Chalak, L., ... & Huang, H. (2019). Structural network maturation of the preterm human brain. Neuroimage, 185, 699-710.
[4] Huang, H., Shu, N., Mishra, V., Jeon, T., Chalak, L., Wang, Z. J., ... & Dong, Q. (2015). Development of human brain structural networks through infancy and childhood. Cerebral Cortex, 25(5), 1389-1404.
[5] Yu, Q., Peng, Y., Kang, H., Peng, Q., Ouyang, M., Slinger, M., ... & Huang, H. (2020). Differential white matter maturation from birth to 8 years of age. Cerebral Cortex, 30(4), 2674-2690.
[6] Huang, H., Jeon, T., Sedmak, G., Pletikos, M., Vasung, L., Xu, X., ... & Mori, S. (2012). Coupling diffusion imaging with histological and gene expression analysis to examine the dynamics of cortical areas across the fetal period of human brain development. Cerebral cortex, 23(11), 2620-2631.
[7] Fornito, A., Arnatkevičiūtė, A., & Fulcher, B. D. (2018). Bridging the gap between connectome and transcriptome. Trends in cognitive sciences, 23(1), 34-50.
[8] Romero-Garcia, R., Atienza, M., Clemmensen, L. H., & Cantero, J. L. (2012). Effects of network resolution on topological properties of human neocortex. Neuroimage, 59(4), 3522-3532.
[9] Li, M., Santpere, G., Kawasawa, Y. I., Evgrafov, O. V., Gulden, F. O., Pochareddy, S., ... & Sestan, N. (2018). Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science, 362(6420).
[10] Eden, E., Navon, R., Steinfeld, I., Lipson, D., & Yakhini, Z. (2009). GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC bioinformatics, 10(1), 1-7.
[11] Raudvere, U., Kolberg, L., Kuzmin, I., Arak, T., Adler, P., Peterson, H., & Vilo, J. (2019). g: Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic acids research, 47(W1), W191-W198.
[12] Huttenlocher, P. R., & Dabholkar, A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. Journal of comparative Neurology, 387(2), 167-178.
Figures