0355
Regional changes in brain development and cognitive outcome in infants with Congenital Heart Disease1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department for Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom, 3Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid and CIBER-BBN, Madrid, Spain, 4Paediatric Cardiology Department, Evelina London Children's Healthcare, London, United Kingdom, 5Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 6Department of Child and Adolescent Psychiatry, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom
Synopsis
Infants with Congenital Heart Disease (CHD) are at high risk of neurodevelopmental disorders. We acquired presurgical neonatal T2-weighted MRI (N=66), cerebral oxygen delivery (CDO2; N=53), and 22-month cognitive and motor scores (N=44). Atypicality indices, representing the degree of deviation of a regional brain volume from the normative neonatal mean for a given gestational age, sex and postnatal age, were calculated. Reduced CDO2 was indirectly associated with lower cognitive scores through the mediating effect of negative bilateral caudate and thalami atypicality indices. The aetiology of cognitive impairments in CHD may encompass poor CDO2 leading to impaired caudate and thalamus growth.
Introduction
Infants with Congenital Heart Disease (CHD) are at risk of neurodevelopmental impairments, the origins of which are currently unclear.1 The aim of this study was to characterise the relationship between neonatal brain development, cerebral oxygen delivery and neurodevelopmental outcome in infants with CHD.Methods
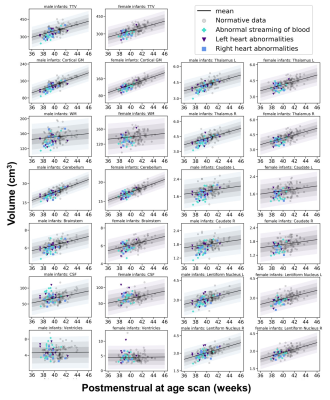
Sixty-six infants [39 male, median (range) gestational age at birth = 38.50 (34.86-41.57) weeks; postmenstrual age at scan median (range) = 39.29 (37.43-42.29) weeks] with serious or critical CHD underwent brain MRI prior to surgery on a 3T MRI scanner situated on the neonatal unit at St Thomas’ Hospital, London. T2-weighted images (voxel size=0.8mm3) were segmented into brain regions using a neonatal-specific algorithm.2,3 We generated normative curves of typical volumetric brain development using Gaussian Process Regression, a Bayesian non-parametric regression technique, implemented in GPy in Python (https://sheffieldml.github.io/GPy/). 219 healthy infants from the Developing Human Connectome Project (dHCP) imaged with the same protocol were used to model typical brain development from 37-45 postmenstrual weeks.4 Atypicality indices (Z), representing the degree of positive or negative deviation of a regional volume from the normative mean for a given gestational age, sex and postnatal age, were calculated for each infant with CHD (Figure 1). Extreme deviations from typical brain development were taken as Z >±2.6 (corresponding to p<0.005). Neonatal cerebral oxygen delivery (CDO2) was calculated from phase contrast angiography in 53 infants with CHD.5 Cognitive and motor abilities were assessed at 22 months (N=44) using the Bayley-Scales of Infant and Toddler Development- 3rd Edition. We assessed the relationship between atypicality indices, CDO2 and cognitive and motor outcome. We also examined whether CDO2 was associated with neurodevelopmental outcome through the mediating effect of regional brain development.6Results
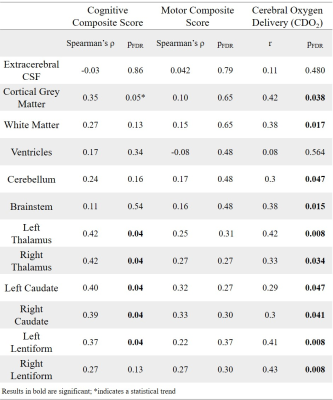
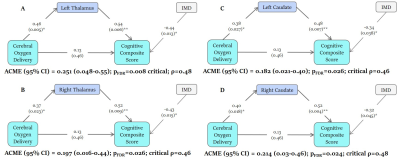
Extreme deviations in development were identified in 13.6% of infants (N=9) with CHD. The most common extreme deviation was enlargement of the extracerebral CSF occurring in 7.6% of babies with CHD. Extreme positive deviations (Z >2.6) were also identified in the ventricles. Extreme negative deviations (Z <-2.6) were identified in the brainstem, bilateral caudate nuclei, left thalamus, cerebellum and total tissue volume.Negative atypicality indices in bilateral caudate nuclei and thalami and left lentiform nucleus were associated with both reduced neonatal CDO2 and poorer cognitive abilities at 22 months across the sample (Table 1). There was a significant indirect relationship between CDO2 and cognition through the mediating effect of lower bilateral caudate and thalami atypicality indices (Figure 2).
Discussion
Previous studies of brain development in this population have assessed differences between infants with CHD and healthy infants at a group level. Here we have mapped brain development in individual infants with CHD to robust normative neonatal data using normative modelling. Using this approach, we identified extreme deviations from typical brain development in over 13% of infants, the most common being increased extracerebral CSF volume, as well as increases in the ventricles and reductions in subcortical structures.Lower cognitive abilities in toddlers with CHD were associated with smaller caudate nuclei, thalami and left lentiform volumes prior to cardiac surgery. Reduced CDO2 was indirectly associated with poor cognitive outcome in early childhood through the mediating effect of reduced caudate and thalamus development. Reduced CDO2 is associated with smaller total brain volume in fetuses7 with CHD and reduced grey matter volume and gyrification are observed in neonates before cardiac surgery.5 Lower basal ganglia and thalamus volumes postoperatively have been associated with lower IQ scores at 6 years.8 Neonatal thalamus and caudate volumes have also been implicated in cognitive abilities in children born prematurely.9,10 Taken together, these data point to the importance of caudate and thalamic development to early cognitive outcome and the potential adverse impact of reduced CDO2 in the neonatal period. Infants with CHD and low subcortical volumes may be at increased risk of impaired cognitive development.
Conclusions
Infants with CHD are at increased risk of extreme deviations in intracranial development, particularly in CSF spaces and subcortical structures, compared to healthy controls. The aetiology of poor cognition in CHD may encompass poor cerebral oxygen delivery leading to impaired caudate and thalamus growth. Interventions to improve cerebral oxygen delivery may promote early brain growth and improve cognitive outcomes in this population.Acknowledgements
This work was supported by the Medical Research Council UK (MR/L011530/1), the British Heart Foundation (FS/15/55/31649), and Action Medical Research (GN2630). The normative sample was collected as part of the Developing Human Connectome Project (dHCP), funded by the ERC grant agreement no. 319456. This research was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z), MRC strategic grant (MR/K006355/1), Medical Research Council Centre grant (MR/N026063/1), and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and Kings College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and social care.References
1. Marino BS, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012; 126: 1143–72.
2. Makropoulos A, et al. Regional growth and atlasing of the developing human brain. Neuroimage 2016; 125: 456–78.
3. Makropoulos A, et al. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 2018; 173: 88–112.
4. Dimitrova R, et al. Individualized characterization of volumetric development in the preterm brain. bioRxiv 2020: 2020.08.05.228700.
5. Kelly CJ, et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci Rep 2017; 7: 15088.
6. Zhang Z, et al. Causal mediation analysis in the context of clinical research. Ann Transl Med 2016; 4: 425
7. Sun L, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015; 131: 1313–23.
8. Claessens NHP, , et al. Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Dev Med Child Neurol 2018; 60: 1052–8.
9. Loh WY, et al. Neonatal basal ganglia and thalamic volumes: very preterm birth and 7-year neurodevelopmental outcomes. Pediatr Res 2017; 82: 970–8.
10. Young JM, et al. Deep grey matter growth predicts neurodevelopmental outcomes in very preterm children. Neuroimage 2015; 111: 360–8.
Figures