0350
Early Non-Contrast Biomarkers of Left Ventricular Cardiomyopathy in Children with Duchenne Muscular Dystrophy1Cardiovascular Institute, Stanford University, Stanford, CA, United States, 2Physics and Biology in Medicine Interdepartmental Program, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Radiology, Stanford University, Stanford, CA, United States, 4Department of Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 5Department of Pediatrics, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Left ventricular(LV) peak mid-wall circumferential strain (Ecc) is a sensitive early biomarker for evaluating the subtle and highly variable onset and progression of cardiomyopathy in pediatric subjects with Duchenne muscular dystrophy (DMD). Cine Displacement Encoding with Stimulated Echoes (DENSE) has proven sensitive to changes in Ecc. Using cine DENSE we show a significantly decreased septal Ecc in LGE negative(-) boys with DMD absent identifiable focal fibrosis compared with controls. A binomial logistic regression model that combines septal Ecc, LV pre-contrast T1, and LV ejection fraction can sensitively distinguish LGE(-) boys with DMD from healthy boys without the need for contrast.
Background
Cardiomyopathy is the leading cause of mortality in boys with Duchenne muscular dystrophy (DMD). Reduced left ventricular (LV) ejection fraction (EF<55%) is a widely used marker of cardiac function and outcomes1, but the decline in LVEF is a relatively late finding among DMD patients. Late gadolinium enhancement (LGE) MRI is the gold standard for detecting focal myocardial fibrosis, but positive LGE is also a late finding in DMD2,3. We seek to define a sensitive non-contrast biomarker to evaluate cardiac involvement in DMD prior to the decline in LVEF or the appearance of LGE.Myocardial native pre-contrast T1 (preT1) mapping can be used to evaluate diffuse fibrosis4 and can offer insight into early cardiac involvement in DMD5. In addition, LV peak mid-wall circumferential strain (Ecc). using tagging is an early biomarker of dysfunction in DMD6-8, but further validation is needed9. Alternatively, Cine Displacement Encoding with Stimulated Echoes (DENSE) is sensitive to changes in Ecc10. Ecc derived from cine DENSE, however, has not been reported in a DMD cohort. Thus, our objectives were: 1) To characterize regional LV Ecc in healthy boys and LGE negative (-) boys with DMD using cine DENSE; and 2) To evaluate a regression model that includes non-contrast biomarkers (Ecc, preT1 mapping, and LVEF) to distinguish between healthy boys and LGE(-) boys with DMD.
Methods
LGE(-) boys with DMD (N=10, 12.5±3.0 years old) and healthy boys (N=12, 13.0±2.0 years old), were prospectively enrolled in an IRB-approved study, consented, and underwent a 3T (Skyra, Siemens) CMR exam. Single-slice, short-axis, mid-ventricular data was acquired using navigator-gated free-breathing cine DENSE (2.5x2.5x8 mm3, TE/TRes=1.2/15ms, ke=0.08cycles/mm, Navg=3, ~2.5min/slice)11. Ecc at each voxel was computed from DENSE images using the open-source DENSE analysis tool12,13. Regional strain analysis was done using a custom DENSE analysis plugin14. Specifically, a mid-ventricular LV slice was segmented into six segments using the AHA model15. Then, septal Ecc was averaged over anteroseptal and inferoseptal segments and lateral Ecc was averaged over inferolateral and anterolateral segments. Two expert clinicians calculated LV and RV volumes and function from standard bSSFP cine images. Average LV preT1 values and RV preT1 values (using a one-pixel centerline approach) were recorded. For each group, within-group differences in the biomarkers of interest (BOI) were tested using a Skillings-Mack test followed by pairwise Wilcoxon signed-rank tests. For each wall segment, group-wise differences in the BOI were tested by a Wilcoxon rank-sum test. Data is reported as median (IQR) and p<0.05 was considered significant. A framework was proposed to determine how all predictors contribute to predicting the significant change in Ecc between healthy boys and LGE(-) boys with DMD (Table 3). Lastly, a binomial logistic regression model tested whether Ecc, LV preT1, and LVEF can distinguish between healthy boys and LGE(-) boys with DMD.Results
LGE(-) boys with DMD were significantly shorter, resulting in significantly smaller BSA compared to healthy controls (Table 1). 4 out of the 10 LGE(-) boys with DMD presented with reduced LVEF (< 55%) and 3 out of the 10 presented with reduced RVEF (<50% 16). Shown in Table 2, LGE(-) boys with DMD had significantly lower RVEDV and RVESV compared to healthy controls. LGE(-) boys with DMD also demonstrated significantly elevated preT1 in LV lateral wall [1336(77) vs 1282(62), p=0.030] and RV wall [1569(184) vs 1434(81), p = 0.008] compared to healthy controls. Depicted in Figure 1, both groups of boys had significantly lower septal Ecc and significantly higher lateral Ecc compared to the Ecc in other segments. LGE(-) boys with DMD exhibited significantly reduced septal Ecc [-0.13(0.01) vs -0.16(0.03), p = 0.019] compared to healthy boys. Table 3 illustrates that LVEF predicts septal Ecc in controls, but not in LGE(-) boys with DMD; while LVEDV and RVEDV predict septal Ecc in LGE(-) boys with DMD, but not in controls. Figure 2 reveals that a combination of septal Ecc, lateral LV preT1, and LVEF best distinguished between LGE(-) boys with DMD and healthy boys, followed by septal Ecc plus LVEF, septal Ecc, and lateral LV preT1 (AUC = 0.92, 0.83, 0.80, and 0.79), respectively.Discussion & Conclusion
LGE(-) boys with DMD and healthy boys both exhibited lower septal Ecc relative to the higher lateral Ecc. Similar findings have been previously observed using tagging in control subjects and DMD patients17,18. The regional heterogeneity in Ecc is likely attributed to regional differences in loading conditions. Similarly, the optimal regression models reveal that decreased LVEDV and RVEDV predict the significantly reduced Ecc in LGE(-) boys with DMD compared with controls, indicating the impaired septal shortening is likely due to a shift in end-diastolic loading conditions for both the LV and RV. Functional changes in the respiratory system owing to DMD may affect the biventricular end-diastolic loading conditions. For distinguishing LGE(-) boys with DMD from healthy boys, septal Ecc outperformed the other individual biomarkers and the combination of septal Ecc, lateral LV preT1, and LVEF outperformed the other biomarker combinations. This combination may provide an early, non-contrast biomarker for detecting the onset of LV cardiac involvement in boys with DMD.Acknowledgements
The authors thank all of the study coordinators, MRI technicians, study
participants, and their families. This study was supported by NIH R01 HL131975
to DBE and NSF DGE 1650604 to NGM.
References
1 Solomon, S. D. et al. Influence of Ejection Fraction on Cardiovascular Outcomes
in a Broad Spectrum of Heart Failure Patients. Circulation 112,
3738-3744, doi:10.1161/circulationaha.105.561423 (2005).
2 Tandon, A. et al. Myocardial fibrosis burden predicts left ventricular
ejection fraction and is associated with age and steroid treatment duration in
Duchenne muscular dystrophy. Journal of
the American Heart Association 4,
e001338 (2015).
3 Silva, M. C. et al. Myocardial delayed enhancement by magnetic resonance
imaging in patients with muscular dystrophy. Journal of the American College of Cardiology 49, 1874-1879 (2007).
4 Everett, R. J. et al. Assessment of myocardial fibrosis with T1 mapping MRI. Clinical Radiology 71, 768-778, doi:https://doi.org/10.1016/j.crad.2016.02.013 (2016).
5 Olivieri, L. J. et al. Native T1 values identify
myocardial changes and stratify disease severity in patients with Duchenne
muscular dystrophy. Journal of
Cardiovascular Magnetic Resonance 18,
72, doi:10.1186/s12968-016-0292-8 (2016).
6 Magrath, P. et al. Cardiac MRI biomarkers for Duchenne muscular dystrophy. Biomarkers in medicine 12, 1271-1289 (2018).
7 Hor, K. N. et al. Circumferential strain analysis identifies strata of
cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance
tagging study. Journal of the American
College of Cardiology 53,
1204-1210 (2009).
8 Ashford, M. et al. Occult cardiac contractile dysfunction in dystrophin-deficient
children revealed by cardiac magnetic resonance strain imaging. Circulation 112, 2462-2467 (2005).
9 Ryan, T. D. et al. Abnormal Circumferential Strain is Present in Young
Duchenne Muscular Dystrophy Patients. Pediatric
Cardiology 34, 1159-1165,
doi:10.1007/s00246-012-0622-z (2013).
10 Wehner, G. J. et al. Validation of in vivo 2D displacements from spiral cine
DENSE at 3T. Journal of Cardiovascular
Magnetic Resonance 17, 5,
doi:10.1186/s12968-015-0119-z (2015).
11 Zhong, X., Spottiswoode, B. S.,
Meyer, C. H., Kramer, C. M. & Epstein, F. H. Imaging three-dimensional
myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI. Magnetic Resonance in Medicine 64, 1089-1097, doi:10.1002/mrm.22503
(2010).
12 Gilliam, A. D., Suever, J. D. &
and contributors. DENSEanalysis,
<Retrieved from https://github.com/denseanalysis/denseanalysis> (2016).
13 Spottiswoode, B. S. et al. Tracking myocardial motion from
cine DENSE images using spatiotemporal phase unwrapping and temporal fitting. IEEE Trans Med Imaging 26, 15-30, doi:10.1109/tmi.2006.884215
(2007).
14 Liu, Z.-Q., Zhang, X. & Wenk, J.
F. Quantification of regional right ventricular strain in healthy rats using 3D
spiral cine dense MRI. Journal of
Biomechanics 94, 219-223, doi:https://doi.org/10.1016/j.jbiomech.2019.07.026
(2019).
15 Cerqueira, M. Standardized
myocardial segmentation and nomenclature for tomographic imaging of the heart:
a statement for healthcare professionals from the Cardiac Imaging Committee of
the Council on Clinical Cardiology of the American Heart Association. Circulation 105, 539–542 (2002).
16 Kawel-Boehm, N. et al. Normal values for cardiovascular magnetic resonance in
adults and children. Journal of
Cardiovascular Magnetic Resonance 17,
29, doi:10.1186/s12968-015-0111-7 (2015).
17 Hor, K. N. et al. Regional Circumferential Strain is a Biomarker for Disease
Severity in Duchenne Muscular Dystrophy Heart Disease: A Cross-Sectional Study.
Pediatric Cardiology 36, 111-119,
doi:10.1007/s00246-014-0972-9 (2015).
18 Ashford, M. W. et al. Occult Cardiac Contractile Dysfunction in
Dystrophin-Deficient Children Revealed by Cardiac Magnetic Resonance Strain
Imaging. Circulation 112, 2462-2467,
doi:doi:10.1161/CIRCULATIONAHA.104.516716 (2005).
Figures
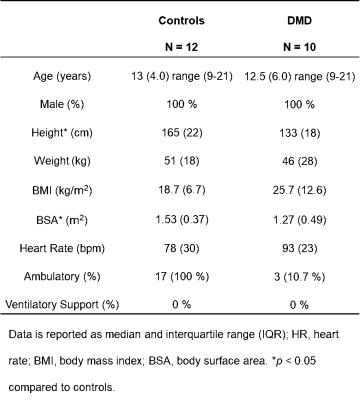
Table 1: Demographics of healthy controls and LGE(-) boys with DMD.
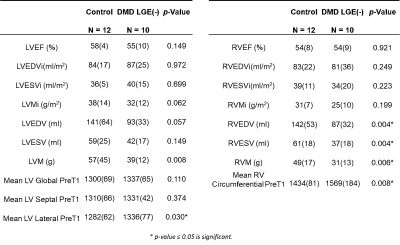
Table 2: Summary of LV and RV volume, function, and pre-contrast T1 values, as
well as differences between LGE(-) patients with DMD and healthy controls.

Table 3: Framework for finding out all predictors contribute to predicting the
septal Ecc in healthy boys and LGE(-) boys with DMD.
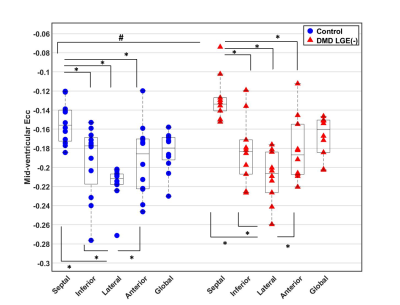
Figure 1: Global and Regional Ecc in
Healthy Boys and LGE(-) boys with DMD. Septal Ecc was significantly lower than anterior, lateral,
and inferior Ecc and the lateral Ecc was significantly higher than septal, anterior,
and inferior Ecc for both the controls or LGE(-) DMD patients. Furthermore, the
LGE(-) DMD patients had significantly reduced septal Ecc compared to healthy
volunteers. *
p-value ≤ 0.05 is significant for a comparison within either controls or LGE(-)
boys with DMD. # p-value
≤ 0.05 is significant for a comparison between controls and LGE(-) patients with
DMD.
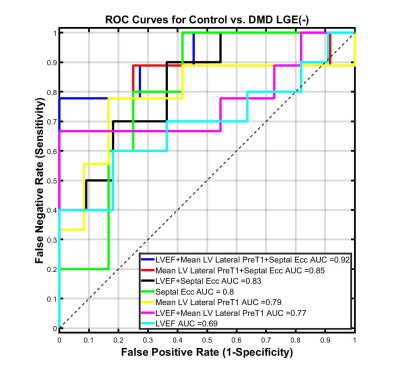
Figure 2: Receiver Operator
Characteristic (ROC) curves for septal Ecc, lateral LV pre-contrast T1, and LVEF
from a binomial logistic regression classifier for distinguishing between LGE(-)
boys with DMD from healthy boys. Larger area under the curve (AUC) values indicate better
classifier performance. Regarding differentiating LGE(-) boys with DMD from healthy
boys, septal Ecc outperforms each individual biomarker and the combinaiton of
septal Ecc, lateral LV pre-contrast T1, and LVEF outperforms the other biomarker
combinations.