0348
Motion Compensated Free-Running 3D Fetal Magnetic Resonance Imaging: Initial Feasibility1Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 3Division of Pediatric Cardiology, Department Woman-Mother-Child, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 4Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 5Signal Processing Laboratory 5 (LTS5), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
A novel framework for 3D MRI of the fetus with retrospective motion compensation is developed and its initial feasibility demonstrated. This approach enables imaging with high isotropic resolution and allows for retrospective evaluation of the complex fetal anatomy in arbitrary scan planes, setting the stage for new possibilities in the assessment of fetal diseases in utero.
Introduction
Magnetic resonance imaging is increasingly used as a complimentary diagnostic tool to ultrasound in the evaluation of the fetus. Substantial progress has been made in the last decade towards MRI-based assessment of the brain, lungs, heart , and abdominal organs in utero (1,2). Existing fetal MRI methods typically trade-off spatial resolution, temporal resolution, and volumetric coverage for abbreviated scan times to reduce image quality degradation caused by maternal and fetal motion. Two-dimensional (2D) imaging methods have been the main driving force behind fetal MRI innovation and to achieve volumetric coverage, a combination of multi-slice, multi-planar acquisitions, and sophisticated motion correction combined with scattered data interpolation and super-resolution algorithms have been used to interrogate the small and complex three-dimensional (3D) geometries of the fetal anatomy (3–7). Nevertheless, 2D acquisitions are susceptible to blur from through-plane motion and suffer from a limited spatial resolution in slice selection direction. Furthermore, they require a high degree of expertise to locate the required scan planes. To address these shortcomings, we propose a novel acquisition and reconstruction framework for 3D fetal MRI with isotropic spatial resolution. Our free-running acquisition uses a large field-of-view (FOV) that encompasses the entire fetus with isotropic spatial resolution, thus simplifying scan planning. Moreover, our reconstruction algorithm retrospectively identifies motion and corrects it. We present here, our initial findings in eight pregnant women in which we quantitatively compare the sharpness of 3D images before and after retrospective motion correction.Methods
Between August and November 2020, eight pregnant women underwent fetal MRI (mean gestational age: 31 weeks, range: 25-36) on a 1.5T MAGNETOM Sola (Siemens Healthcare, Erlangen, Germany) after ultrasound detection of a fetal malformation or a placental abnormality. In compliance with our institutional guidelines, all patients provided consent for an additional research scan performed after the diagnostic sequences. A prototype free-running (8,9) slab-selective 3D bSSFP sequence with radial phyllotaxis sampling (10) was acquired in all subjects during free-breathing with an isotropic FOV: (256 mm)3, isotropic spatial resolution: 1 mm3, TR/TE: 4.04/2.02 ms, number of radial profiles: 88590, and total acquisition time: 6 minutes. Each data set was reconstructed offline using the following framework: First, low spatial resolution (4 mm) 3 “real-time” images with 500 ms temporal resolution were reconstructed from the central 64 k-space coefficients of N consecutive radial profiles and regularized along the temporal dimension using k-t sparse SENSE (11). Second, clustering of the real-time image series was performed using a recently proposed method called SImilarity-driven Multi-dimensional Binning Algorithm (SIMBA) to identify consistent “motion-states”, effectively freezing unique periods of maternal and fetal motion (12). Third, the original full-resolution data was binned according to the identified motion states. Fourth, the unique motion states were co-registered using NiftyReg (13) to produce a final high-quality motion compensated 3D image. To evaluate our proposed framework, images were qualitatively evaluated for their ability to identify and correct motion. Image sharpness was measured before and after motion correction by using manually selected regions of interest in the brain (14). Sharpness results were statistically compared using a paired t-test.Results
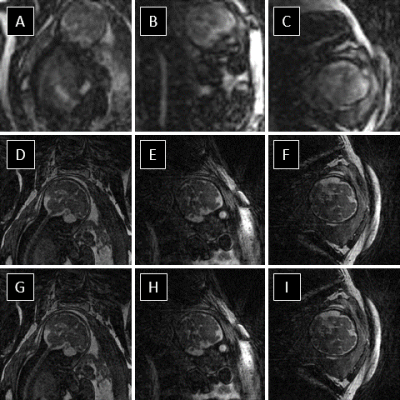
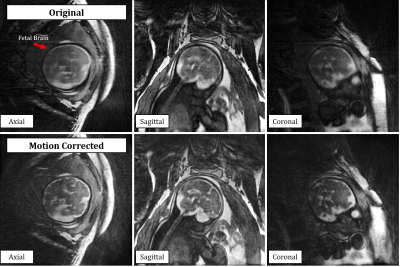
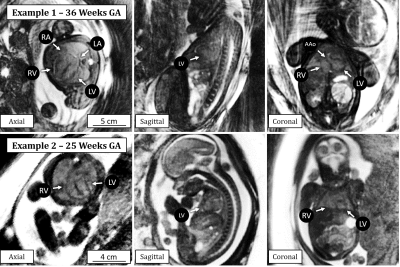
The reconstruction of real-time 3D image series provided retrospective identification of maternal respiration and gross fetal movement in all subjects (Fig 1. a-c). Using the real-time images, clustering of the original data into unique motion-states (Fig 1. d-e) provided excellent depiction of both maternal and fetal movement and subsequent registration of these images was successful in all subjects (Fig. 1 f-h). The effect of our motion correction strategy on image quality is well demonstrated in Fig. 2 wherein a noticeable increase in the sharpness of fetal brain structures is shown. Additionally, the small structures of the fetal heart could be visualized and retrospectively reformatted in arbitrary planes due to the isotropic 3D acquisition. Finally, the quantitative evaluation of image sharpness yielded statistically significant improvement when comparing image reconstructions pre- and post-motion correction (2.6 ± 0.7 vs 3.5 ± 0.8, t-test p < 0.05).Discussion and Conclusion
This work establishes the initial feasibility of a motion-robust 3D fetal MRI acquisition and reconstruction framework with millimetric isotropic spatial resolution that can capture the entire fetal anatomy with minimal scan planning effort. While preliminary quantitative analysis demonstrated significant improvements in the image sharpness using our proposed framework, further comparison to conventional 2D fetal MRI imaging techniques as well as a comparison with the gold standard ultrasound is required to assess the clinical utility of this new methodology. Nevertheless, imaging the entire fetus at high isotropic spatial resolution, as demonstrated here, may create new opportunities for improved assessment of the fetal brain, heart, lungs, and abdominal organs in utero, further solidifying the complimentary nature of MRI in cases where ultrasound may be limited by different factors, including multiple pregnancies, acoustic shadowing from fetal and maternal bone, maternal obesity, and oligohydramnios, among others.Acknowledgements
No acknowledgement found.References
1. Gholipour A, Estroff JA, Barnewolt CE, et al. Fetal MRI: A Technical Update with Educational Aspirations. Concepts Magn. Reson. Part A. Bridg. Educ. Res. 2014;43:237–266 doi: 10.1002/cmr.a.21321.
2. Roy CW, van Amerom JFP, Marini D, Seed M, Macgowan CK. Fetal Cardiac MRI: A Review of Technical Advancements. Top. Magn. Reson. Imaging 2019;28:235–244 doi: 10.1097/RMR.0000000000000218.
3. Rousseau F, Glenn OA, Iordanova B, et al. Registration-Based Approach for Reconstruction of High-Resolution In Utero Fetal MR Brain Images. Acad. Radiol. 2006;13:1072–1081 doi: 10.1016/j.acra.2006.05.003.
4. Gholipour A, Estroff JA, Warfield SK. Robust Super-Resolution Volume Reconstruction From Slice Acquisitions: Application to Fetal Brain MRI. IEEE Trans. Med. Imaging 2010;29:1739–1758 doi: 10.1109/TMI.2010.2051680.
5. Amerom JFP, Lloyd DFA, Deprez M, et al. Fetal whole‐heart 4D imaging using motion‐corrected multi‐planar real‐time MRI. Magn. Reson. Med. 2019:mrm.27798 doi: 10.1002/mrm.27798.
6. Lloyd DFA, Pushparajah K, Simpson JM, et al. Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: a prospective, single-centre cohort study. Lancet 2019 doi: 10.1016/S0140-6736(18)32490-5.
7. Tourbier S, Bresson X, Hagmann P, Thiran J-P, Meuli R, Cuadra MB. An efficient total variation algorithm for super-resolution in fetal brain MRI with adaptive regularization. Neuroimage 2015;118:584–597 doi: 10.1016/j.neuroimage.2015.06.018.
8. Coppo S, Piccini D, Bonanno G, et al. Free-running 4D whole-heart self-navigated golden angle MRI: Initial results. Magn. Reson. Med. 2015;74:1306–1316 doi: 10.1002/mrm.25523.
9. Di Sopra L PD, Coppo S, Stuber M YJ. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn Reson Med. 2019;00:1:15.
10. Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO. Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI. Magn. Reson. Med. 2011;66:1049–56 doi: 10.1002/mrm.22898.
11. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007;58:1182–1195 doi: 10.1002/mrm.21391.
12. Heerfordt J, Whitehead KK, Bastiaansen JAM, et al. Free-running SIMilarity-Based Angiography (SIMBA) for simplified anatomical MR imaging of the heart. 2020. https://arxiv.org/abs/2007.06544
13. Modat M, Ridgway GR, Taylor ZA, et al. Fast free-form deformation using graphics processing units. Comput. Methods Programs Biomed. 2010;98:278–284 doi: 10.1016/j.cmpb.2009.09.002.
14. Blanchet G, Moisan L. An explicit sharpness index related to global phase coherence. In: 2012 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP). IEEE; 2012. pp. 1065–1068. doi: 10.1109/ICASSP.2012.6288070.
Figures