0347
Rapid fetal HASTE imaging using variable flip angles and simultaneous multislice wave-LORAKS1Massachusetts Institute of Technology, Cambridge, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Department of Electrical Engineering, University of Southern California, Los Angeles, CA, United States, 4Boston Children’s Hospital, Boston, MA, United States, 5Harvard Medical School, Boston, MA, United States, 6Fetal-Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, Boston, MA, United States, 7Department of Radiology, Harvard Medical School, Cambridge, MA, United States, 8Harvard-MIT Health Sciences and Technology, Cambridge, MA, United States, 9Institute for Medical Engineering and Science, Cambridge, MA, United States
Synopsis
Fetal MRI utilizes Half-Fourier-acquisition-single-shot-turbo-spin-echo (HASTE) for rapid T2-weighted imaging to mitigate motion. However, specific-absorption-rate (SAR) constraints from the refocusing pulse train reduce acquisition efficiency. Variable refocusing flip angle (VFA) acquisitions can improve efficiency, but may suffer from low signal-to-noise ratios (SNR). Here, we propose a VFA scheme and incorporate a rapid, low-SAR calibration scan. We simulate and prospectively evaluate the SNR and SAR properties of the VFA scheme and utilize the calibration scan for LORAKS parallel imaging and retrospective evaluation of wave-encoded simultaneous-multislice (SMS). VFA prospectively reduces acquisition time by ~2.3-2.5x and incorporating SMS could further improve efficiency.
Introduction
Fetal MRI utilizes Half-Fourier-acquisition-single-shot-turbo-spin-echo (HASTE) for motion robust, rapid T2-weighted imaging.1 Typically, HASTE encodes a slice in ~0.5 seconds (Rin-plane=2) but specific-absorption-rate (SAR) constraints from the train of high flip angle refocusing pulses necessitate ~1.5 seconds between slices, a 3x loss in efficiency.Variable refocusing flip angles (VFA) reduce SAR while maintaining image quality.2-5 VFA has been employed in a fetal setting,6 but suffered from low signal-to-noise ratios (SNR).
We propose an alternative VFA scheme2 and incorporate a low-SAR external calibration scan to improve acquisition efficiency in fetal MRI. Simulations evaluate SAR and SNR of the proposed VFA scheme, prospective phantom and in-vivo experiments compare standard HASTE to our proposed scheme reconstructed using an external calibration scan, and retrospective experiments illustrate the potential of SMS-wave encoded acquisitions with LORAKS reconstruction.7-11 VFA prospectively reduces acquisition time by ~2.3x and incorporating SMS could further improve efficiency.
Methods
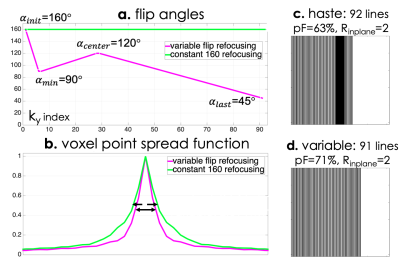
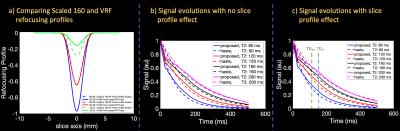
Standard clinical HASTE acquires 92 ky lines (511ms readout duration) with 63% partial Fourier, TE = 122 ms, 24 auto-calibration lines, Rin-plane = 2, and 160 degree refocusing pulses. VFA sets 4 control angles to specify the train2,3 and acquires 91 lines (505ms readout duration) with 71% partial Fourier, TE = 155 ms, no calibration region, and Rin-plane = 2. Figure 1 visually compares the described HASTE and VFA acquisitions. A rapid, low-SAR GRE calibration scan obviates the need for integrated ACS data, and the proposed regime achieves 2.94x lower SAR and 1.36x reduction in voxel blurring.Figure 2a compares the refocusing profile of the 160° pulse scaled to different VFA's and the profile generated from the scaled VFA HASTE pulse using RF-Tools.12 For angles greater than 90°, VFA refocusing profiles match the scaled 160° profile up to 5%. Smaller angles later in the train match between 10 - 30%, but potential effects are minimal due to signal decay.
Figure 2b and 2c compares HASTE and VFA signal evolution simulated using an isochromat-based Bloch, Carr-Purcell-Meiboom-Gill simulation for T2-values expected in the fetal brain and T1 = 1 s,13,14 with and without slice profile effects. Assuming SNR is proportional to signal strength at the TE, VFA yields between 82-90% SNR relative to HASTE in both cases.
Experiments
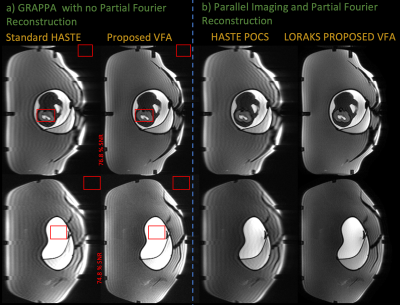
We acquired data using the standard HASTE and VFA schemes with 350x350 mm FOV and 1.36x1.36x3 mm voxels on both an anthropomorphic fetal phantom15 and a pregnant mother who signed an informed consent form approved by our institution’s IRB. In both cases, a rapid GRE calibration scan was also acquired immediately before the HASTE readouts.We compare GRAPPA16 reconstructions on the standard HASTE acquisition using integrated calibration lines and the proposed acquisition using external references and compute SNR by dividing averaged regions inside and outside the object.
We also compare GRAPPA with projection-onto-convex-sets (POCS) partial Fourier on the HASTE acquisition to SENSE-LORAKS reconstructions on the proposed VFA acquisition.17-19 To illustrate constrained reconstructions with external references, we chose LORAKS to take advantage of phase smoothness to estimate missing portions of k-space.
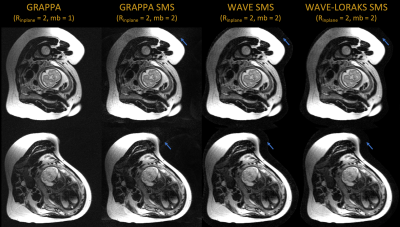
For some pregnant mothers, including the one that participated in our study, our prescribed minimum TR of 600 ms operates at 70-75% of the SAR limit. In cases with remaining SAR budget, SMS encoding could further improve sequence efficiency between 1.25-1.75x. To evaluate proof-of-concept SMS, we perform retrospective multiband=2, Rin-plane=2, wave-encoded SMS experiments on the proposed in-vivo VFA data.
We generate wave-encoded SMS data by applying the wave point-spread-function to the acquired multi-channel data and then collapsing the FOV-shifted data across slices.8,9 We reconstruct with and without LORAKS regularization using the generalized SENSE-forward model that uses Fourier encoding, coil sensitivities, and wave point spread functions to map multislice images to k-space.10 We modeled wave-encoded SMS data with maximum gradient amplitudes of 10.2 mT/m, 4 cycles, maximum slew rate of 180 mT/m/ms, FOV shift of 3,7 and 51 mm slice distance. Coil sensitivity maps and slice-GRAPPA kernels are calibrated from the external calibration scans.
For mothers, where the VFA acquisition may operate at 100% SAR (providing ~3x improvement in efficiency), further undersampling enabled by the aforementioned techniques could reduce SAR budgets to enable SMS efficiency.
Results
Figure 3a compares GRAPPA reconstructions between standard HASTE and the proposed technique in a fetal phantom. VFA reduces image blurring, maintains qualitatively similar contrast, and retains ~75% SNR in comparison to HASTE. Figure 3b illustrates how external calibration enables SENSE-LORAKS reconstructions.Figure 4 performs the above comparisons on prospectively acquired in-vivo data. The proposed technique retains 83% SNR and reduces blurring. SENSE-LORAKS produces qualitatively clean images using the external reference scan as calibration. Note, the proposed technique acquired the entire stack of slices 2.3x faster than HASTE.
Figure 5 shows retrospective wave-encoded SMS reconstructions. While slice-GRAPPA incurs some noise amplification, wave-SMS with LORAKS produces relatively clean images. All reconstructions were performed with external reference calibration.
Conclusions
We present a VFA train and sampling scheme which provides a 2.3x reduction in imaging time and retains SNR in prospective phantom and in-vivo experiments. Additionally, incorporating GRE reference scans enable advanced reconstruction techniques such as LORAKS and wave-encoded SMS. We plan to implement prospective wave-SMS, acquire reference scans after each slice-group to mitigate motion effects, and evaluate a larger cohort of subjects.Acknowledgements
This work was supported in part by research grants NIH R01 EB017337, U01 HD087211, R01HD100009, R01 EB028797, U01 EB025162, P41 EB030006, U01 EB026996 and the NVidia Corporation for computing support. In addition, this material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1122374. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.References
1. Patel MR, Klufas RA, Alberico RA, Edelman RR. Half-fourier acquisition single-shot turbo spin-echo (HASTE) MR: comparison with fast spin-echo MR in diseases of the brain. AJNR Am J Neuroradiol. 1997;18: 1635–1640.
2. Loening AM, Saranathan M, Ruangwattanapaisarn N, Litwiller DV, Shimakawa A, Vasanawala SS. Increased speed and image quality in single-shot fast spin echo imaging via variable refocusing flip angles. J Magn Reson Imaging. 2015;42: 1747–1758.
3. Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55: 1030–1037.
4. Chen F, Zhang T, Cheng JY, Shi X, Pauly JM, Vasanawala SS. Autocalibrating motion-corrected wave-encoding for highly accelerated free-breathing abdominal MRI. Magn Reson Med. 2017;78: 1757–1766.
5. Chen F, Cheng JY, Taviani V, Sheth VR, Brunsing RL, Pauly JM, et al. Data‐driven self‐calibration and reconstruction for non‐cartesian wave‐encoded single‐shot fast spin echo using deep learning. J Magn Reson Imaging. 2019;6: 698.
6. Yamin Arefeen, Borjan Gagoski, Esra Turk, P. Ellen Grant, Jacob White, Kawin Setsompop, and Elfar Adalsteinsson. Single-shot T2 Fetal MRI with variable flip angles and full k-space sampling with nonlinear inversion: towards improved SAR and sharpness. Proceedings of The International Society for Magnetic Resonance in Medicine 2020 Scientific Meeting.
7. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67: 1210–1224.
8. Gagoski BA, Bilgic B, Eichner C, Bhat H, Grant PE, Wald LL, et al. RARE/turbo spin echo imaging with simultaneous multislice Wave-CAIPI. Magn Reson Med. 2015;73: 929–938.
9. Bilgic B, Gagoski BA, Cauley SF, Fan AP, Polimeni JR, Grant PE, et al. Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med. 2015;73: 2152–2162.
10. Kim TH, Bilgic B, Polak D, Setsompop K, Haldar JP. Wave‐LORAKS: Combining wave encoding with structured low‐rank matrix modeling for more highly accelerated 3D imaging. Magn Reson Med. 2019;81: 1620–1633.
11. Bo Zhao, Borjan Gagoski, Justin P. Haldar, Elfar Adalsteinsson, Ellen Grant, and Lawrence L. Wald. A Subspace Approach to Accelerated HASTE Acquisition for Fetal Brain MRI. Proceedings of The International Society for Magnetic Resonance in Medicine 26th Scientific Meeting.
12. Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm (NMR imaging). IEEE Trans Med Imaging. 1991;10: 53–65.
13. Ben-Eliezer N, Sodickson DK, Block KT. Rapid and accurate T2mapping from multi-spin-echo data using Bloch-simulation-based reconstruction. Magnetic Resonance in Medicine. 2015. pp. 809–817. doi:10.1002/mrm.25156
14. Jeffrey N Stout, Congyu Liao, Esra Abaci Turk, Borjan Gagoski, P. Ellen Grant, Lawrence L. Wald, and Elfar Adalsteinsson. T1 and T2 mapping of the placenta and fetal brain with magnetic resonance fingerprinting (MRF) during maternal hyperoxia. In Proceedings of the 27th Annual Meeting of ISMRM. : 1182.
15. Pablo Garcia-Polo, Borjan Gagoski, Bastien Gurrein, Eric Gale, Elfar Adalsteinsson, Ellen Grant, Lawrence Wald. An anthropomorphic MR phantom of the gravid abdomen including the uterus, placenta, fetus and fetal brain. In Proceedings of ISMRM 2015 1545. 2015.
16. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47: 1202–1210.
17. Haldar JP. Autocalibrated loraks for fast constrained MRI reconstruction. 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI). 2015. pp. 910–913.
18. Haldar JP, Zhuo J. P-LORAKS: low-rank modeling of local k-space neighborhoods with parallel imaging data. Magn Reson Med. 2016;75: 1499–1514.
19. Kim TH, Setsompop K, Haldar JP. LORAKS makes better SENSE: Phase-constrained partial fourier SENSE reconstruction without phase calibration. Magnetic Resonance in Medicine. 2017. pp. 1021–1035. doi:10.1002/mrm.26182
Figures