0341
Fast High-Resolution 19F-MRSI of Perfluorocarbon Nanoemulsions for MRI Cell Tracking Using SPICE with Learned Subspaces1Department of Electrical and Computer Engineering, University of Illinois, Urbana-Champaign, Urbana, IL, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois, Urbana-Champaign, Urbana, IL, United States, 3Animal Imaging Center, University of Pittsburgh, Pittsburgh, PA, United States, 4Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA, United States, 5Graduate School of Pharmaceutical Sciences, Duquesne University, Pittsburgh, PA, United States
Synopsis
19F-MRSI has the potential to track multiple perfluorocarbon nanoemulsions simultaneously, but existing 19F-MRSI schemes have been limited to CSI, which provides a poor tradeoff between resolution and scan time. In this work, a novel method is proposed to enable fast high-resolution 19F-MRSI. In the proposed method, (k,t)-space is sampled rapidly in EPSI trajectories; data processing is accomplished using a union-of-subspaces model with pre-learned spectral basis. The proposed method has been evaluated using simulation and experimental data, producing encouraging results. The proposed method may open up new opportunities for simultaneous tracking of different labeled cell populations in vivo.
Introduction
Perfluorocarbon (PFC) nanoemulsions (NEs) can be used to label and track cells in vivo.1,2 19F-MRSI has been recognized as a potentially powerful tool for multi-color imaging of PFC-labeled cells because of negligible background signals, high biocompatibility and unique spectral patterns of different PFCs.3-5However, unlike 1H chemical shifts, 19F chemical shift dispersion is large, stretching out over 300 ppm.6 Such a large chemical shift range makes it difficult to implement spatiotemporal sampling trajectories (e.g., EPSI and spiral trajectories) without spectral aliasing, especially in high magnetic field scanners. Besides, it can also introduce chemical shift artifacts along the readout direction.7 Therefore, existing 19F-MRSI methods have been limited to CSI-type data acquisition schemes, which provide a poor tradeoff between spatial resolution and scan time. Although some methods have been proposed to accelerate 19F CSI, like compressed sensing,8,9 the current capability is still rather limited.
This work proposes a new method to enable fast high-resolution 19F-MRSI using SPICE. With a union-of-subspaces model and pre-learned spectral prior information,10,11 the proposed method is able to reconstruct high-quality spatiospectral distributions from highly undersampled EPSI data. The proposed method has been validated using both simulation and experimental data, producing very encouraging results.
Methods
Imaging modelWe represent the desired 19F-MRSI signal using the union-of-subspaces model12:
$$\rho(\boldsymbol{x},f)=\sum_{m=1}^M\sum_{\ell=1}^{L_m}c_{m,\ell}(\boldsymbol{x})v_{m,\ell}(f),$$
where $$$\{v_{m,\ell}(f)\}_{\ell=1}^{L_m}$$$ is a set of spectral basis functions for the $$$m$$$-th molecule and $$$\{c_{m,\ell}(\boldsymbol{x})\}_{\ell=1}^{L_m}$$$ are the corresponding spatial coefficients. The spectral basis functions can be pre-learned from high-quality training data as in previous works.10,11 In 19F-MRSI studies, $$$\rho(\boldsymbol{x},f)$$$ is composed of signals from the PFC molecules used in the specific study, for example, perfluoro-15-crown-5-ether (PCE) and perfluorooctyl bromide (PFOB). The union-of-subspaces model effectively reduces the number of degrees-of-freedom for representing the desired spatiospectral distributions, relaxing the sampling requirements for accurate recovery of the underlying spatiospectral function.
Data acquisition
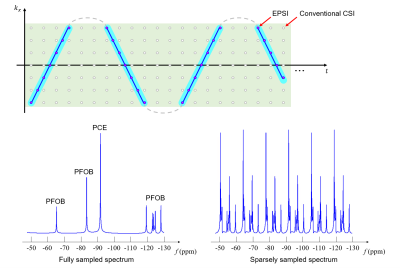
Enabled by the union-of-subspaces model, a 3D EPSI acquisition scheme was proposed. The data acquisition scheme, illustrated in Fig. 1, has the two key features: (a) extended k-space coverage in EPSI trajectories to achieve high spatial resolution, and (b) highly sparse temporal sampling to reduce data acquisition time. This scheme offers high spatiospectral encoding efficiency, yielding a factor of ~40 acceleration over the conventional CSI scheme, but the data thus acquired have a 6-fold spectral undersampling and cover (k,t)-space in tilted trajectories, which require special data processing scheme to avoid aliasing and chemical shift artifacts.
Data processing
Reconstruction of the desired spatiospectral functions was accomplished using a model-based method. The proposed method has two key features: (a) explicit incorporation of sparse and non-uniform (k,t)-space sampling patterns in the imaging model for effective correction of chemical shift artifacts, and (b) use of spectral prior information in the form of pre-learned spectral basis for separation of different molecules from undersampled data. Note that after processing, artifact-free spatiospectral function of each molecule can be obtained. In other words, the proposed method performs reconstruction and spectral quantification directly from the (k,t)-space data.
More specifically, the spatial coefficients of each molecule were estimated from the acquired data, $$$d(\boldsymbol{k},t)$$$, by solving the following optimization problem:
$$\{\hat{c}_{m,\ell}(\boldsymbol{x})\}=\arg\min_{\{c_{m,\ell}(\boldsymbol{x})\}}\left\|d(\boldsymbol{k},t)-\Omega(\boldsymbol{k},t)\mathcal{F}_{\boldsymbol{x}}\mathcal{F}_{f}B_0\left\{\sum_{m=1}^{M}\sum_{\ell=1}^{L_m}c_{m,\ell}(\boldsymbol{x})v_{m,\ell}(f)\right\}\right\|_2^2+\lambda \sum_{m=1}^{M}\sum_{\ell=1}^{L_m}\|\Phi\left\{c_{m,\ell}(\boldsymbol{x})\right\}\|_1,$$
where $$$\Omega(\boldsymbol{k},t)$$$ denotes the sparse and non-uniform sampling operator in (k,t)-space, $$$B_0$$$ the phase term caused by the field inhomogeneity, $$$\mathcal{F}_{\boldsymbol{x}}$$$, $$$\mathcal{F}_{f}$$$ Fourier encoding along spatial and spectral directions, respectively, and $$$\Phi$$$ the total variation transform. After the spatial coefficients were estimated, the desired spatiospectral distribution of each molecule can be reconstructed as:$$\hat{\rho}_m(\boldsymbol{x},f)=\sum_{\ell=1}^{L_m}\hat{c}_{m,\ell}(\boldsymbol{x})v_{m,\ell}(f).$$
Results
SimulationA set of simulation results is shown in Fig. 2. Subspace-based quantification separated PCE and PFOB molecules well from sparsely sampled data. However, non-uniform (k,t)-space sampling caused chemical shift artifacts. By incorporating the non-uniform sampling into the model, accurate reconstruction and quantification results were obtained.
Phantom
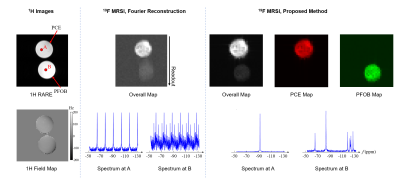
Phantom experiments were performed on a phantom with two vials filled with NEs of PCE and PFOB on a 9.4T Bruker AV3HD scanner equipped with a BGA-12S HP gradient set, a dual-tuned 1H/19F 40-mm birdcage resonator and ParaVision 6.0.1. The data acquisition parameters were: $$$\mathrm{FOV}=40\times40\times80\mathrm{~mm}^3$$$, $$$\mathrm{FA}=15^\circ$$$, $$$\mathrm{TR/TE}=800/0.2\mathrm{~ms}$$$, spatial matrix size $$$=40\times40\times40$$$ and total data acquisition time $$$=21.3$$$ min. A set of representative phantom results is shown in Fig. 3. The Fourier reconstructed PFOB signals suffered from off-resonance chemical shift artifacts, and the spectra suffered from aliasing artifacts. The proposed method produced high-quality PCE and PFOB maps and spectra without chemical shift or aliasing artifacts. It would require more than 14 hours to obtain the same results using conventional CSI schemes.
Biological phantom
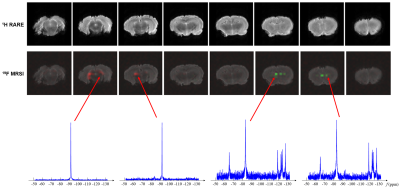
PCE and PFOB NEs were injected into a fixed rat brain and EPSI data were acquired using the parameters similar to the phantom experiment. Figure 4 shows the results obtained using the proposed method. As can be seen, high-quality PCE and PFOB spatiospectral functions were obtained using the proposed method. The spatial distributions of PCE and PFOB matched our expectation.
Conclusions
A new subspace-based approach has been proposed for fast high-resolution 19F-MRSI, with an unprecedented capability of $$$1\times1\times2\mathrm{~mm^3}$$$ spatial resolution in 21.3 min. Simulation and experimental results have demonstrated the feasibility of obtaining high-quality maps of multiple PFC NEs using the proposed method. With further development, this method may provide a powerful tool for in vivo multi-color labeled cell imaging.Acknowledgements
The authors gratefully acknowledge Dr. Johannes Schneider from Bruker BioSpin for advice and assistance implementing the 3D EPSI sequence.References
1. Partlow KC, Chen J, Brant JA et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007; 21(8): 1647-1654.
2. Srinvas M, Heerschap A, Ahrens ET, Figdor CG and de Vries IJM. 19F MRI for quantitative in vivo cell tracking. Trends Biotechnol. 2010; 28(7): 363-370.
3. Stoak CH, Hees PS, Huang HN et al. A new perfluorocarbon for use in fluorine‐19 magnetic resonance imaging and spectroscopy. Magn Reson Med. 1993; 29(2): 188-195.
4. Ruiz-Caballo J, Bernett BP, Bottomley PA and Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011; 24(2): 114-129.
5. Jacaby C, Temme S, Mayenfels F et al. Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half‐lives and sensitivity. NMR Biomed. 2014; 27(3): 261-271.
6. de Graaf RA, In vivo NMR spectroscopy: principles and techniques. Hoboken, NJ: John Wiley and Sons, 2007.
7. Dwyer AJ, Knop RH and Hoult DI. Frequency shift artifacts in MR imaging. J Comput Assist Tomogr. 1985; 9(1); 16-18.
8. Kampf T, Fisher A, Basse- Lüsebrink TC et al. Application of compressed sensing to in vivo 3D 19F CSI. J. Magn. Reson. 2010; 207(2): 262-273.
9. Zhong J, Mills PH, Hitchens TK and Ahrens ET. Accelerated fluorine‐19 MRI cell tracking using compressed sensing. Magn Reson Med. 2013; 69(6): 1683-1690.
10. Li Y, Lam F, Clifford B and Liang Z-P. A subspace approach to spectral quantification for MR spectroscopic imaging. IEEE Trans Biomed Eng. 2017; 64: 2486-2489.
11. Lam F, Li Y, Guo R, Clifford B and Liang Z-P. Ultrafast magnetic resonance spectroscopic imaging using SPICE with learned subspaces. Magn Reson Med. 2020; 83: 377-390.
12. Ma C, Lam F, Johnson CL and Liang Z-P. Removal of nuisance signals from limited and sparse 1H MRSI data using a union-of-subspaces model. Magn Reson Med. 2016;75(2):488‐497.
Figures