0333
MRzero with dAUTOMAP reconstruction– automated invention of MR acquisition and neural network reconstruction1Neuroradiology, University Clinic Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Magnetic Resonance Center, Max-Planck Institute for Biological Cybernetics, Tübingen, Germany, 3Empirical Inference, Max-Planck Institute for Intelligent Systems, Tübingen, Germany, 4Pattern Recognition Lab, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 5Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tübingen, Germany
Synopsis
We present an end-to-end optimized T1 mapping utilizing MRzero - a fully differentiable Bloch-equation-based MRI sequence invention framework. A convolutional neural network is employed for combined image reconstruction and parameter mapping. The pipeline performs a joint optimization of sequence parameters and neural network parameters to create a full autoencoder for T1 mapping. We demonstrate for in vivo measurements at 3T, that the CNN based reconstruction and T1 mapping outperformes a conventional reconstruction with pixelwise neural network based T1 quantification.
Introduction
We propose a supervised learning approach for automated generation of MR sequences and subsequent image reconstruction. In this work, we extend previous approaches1 by using a convolutional neural network for combined image reconstruction and parameter mapping to create a full autoencoder for T1 mapping . The autoencoder performs joint optimization of sequence parameters and neural network parameters of the dAUTOMAP approach2.Methods
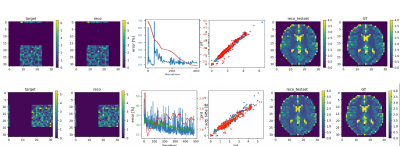
The fully differentiable MRI pipeline is simulated end-to-end with Bloch parameters as input and T1 values as target. A detailed description of the MRzero optimization pipeline can be found in 1. The used MR sequence is based on an inversion prepared 2D FLASH sequence with matrix size 32x32, TR=15ms, TE=8ms, FA=5deg, FOV=200mm, repeated 6 times with varying TI and Trec. Together with the neural network parameters, all TI/Trec times are optimized to find an optimized sequence for T1 mapping. Two different sequence optimization strategies are investigated. For the first approach, the sequence parameter TI and Trec are initialized to values used in a standard inversion recovery protocol for T1 mapping. During optimization, an additional penalty for delays is applied to enforce shorter acquisition times. In the second approach, TI and Trec times are set to zero, also only the first gradient echo readout is prepared with an inversion pulse. No time restriction is applied for this case.The forward process and the architecture of the CNN used for image reconstruction and T1 mapping is shown in Figure 1. It is based on a decomposed AUTOMAP (dAUTOMAP) architechure2. The network is pretrained on a training dataset simulated with the initial sequence parameters. The T1 training dataset consists of 10,000 T1 maps with matrix size 32x32. For each target sample, a rectangle with matrix size 16x16 at varying spatial location with voxel-wise randomly assigned PD, T1, T2 and B0 is defined.
For joint optimization of sequence parameters and NN parameters, at each iteration a new training sample is generated by simulation with the updated sequence parameters. In total 500 iterations are performed for each optimization strategy.
The initial and final optimized sequences are exported using pulseq3 for in vivo measurements performed on a PRISMA 3T scanner (Siemens Healthineers, Erlangen Germany), using a 20 channel head coil.
Results
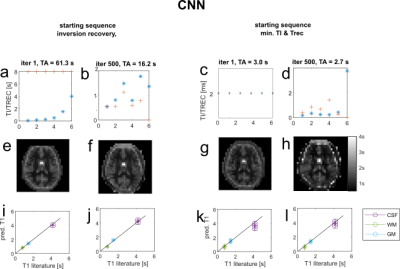
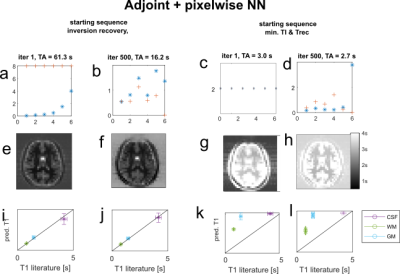
Figure 2 shows the error curves during training and one training/validation sample pair for the final iteration. Figure 3a,b shows the sequence parameter outcome for the first approach, which was initialized to a standard inversion recovery sequence. The total acquisition time could be reduced from 63.3s to 16.2s, while largely preserving image quality. Optimized TI/Trec times range from 0.5s to 1.8s and 0.5s to 1.1s, respectively. Figure 3c,d shows sequence parameters for the second approach, which was initialized with minimal TI=Trec=2ms. Here, acquisition time stays below 3s, even without explicit time restriction. Optimized TI and Trec times range from 0.4ms to 5.1ms and 0.5ms to 4.8s, respectively. T1 maps of a healthy subject generated by the initial and final optimized sequences are displayed in Figure 3e,f,g,h. For both cases, T1 predictions closely agree with the inversion recovery result and literature values at 3T4. Interestingly, this holds even for very short times in the second case (Figure 3c). This is a unique feature of the CNN-based reconstruction. To confirm this, we reconstructed the images conventionally with the adjoint encoding operator formalism1, and trained a pixel-wise network to map to T1. The results are shown in Figure 4; also for the pixel-wise network, both the standard inversion recovery and the corresponding optimized sequence provide T1 maps, which match aswell to literature values. However, for sequence optimization from zero the conventional reconstruction with pixel-wise T1 Mapping results in strong overestimation of T1 values (Figure 4g,h).Discussion
The conducted experiments showed that a combined reconstruction and T1 mapping using a CNN outperforms conventional reconstruction with subsequent NN based T1 quantification especially for very short time look-locker5 like sequences. Using a CNN for reconstruction and T1 mapping allows incorporating spatial information like blurring and partial volume effects into the neural network learning. This additional information may lead to better performances compared to pixelwise T1 quantification. However, by using convolutional layers, the network becomes resolution dependent and therefore needs to be retrained for different image sizes. The proposed approach can be scaled to higher resolution, as it is only limited by computation time. For this abstract only a small resolution could be employed, as the computation time for one sequence optimization was about 2 days on a GPU, but higher resolutions are currently in training.Conclusion/Outlook
In this work, we proposed a joint optimization of sequence parameters and neural network parameters for T1 mapping using a convolutional neural network for combined image reconstruction and T1 quantification. We demonstrated the advantage of CNN/dAUTOMAP over a pixelwise NN with conventional reconstruction. The T1 optimization pipeline provides the first steps towards multi-parametric mapping - similar to MR fingerprinting - yielding PD, T1, and T2, as well as B1 and B0 inhomogeneity maps. Image resolution will be improved in the future, mainly limited by long computation times. An approach to accelerate computation time by utilizing an analytical signal equation instead of a full Bloch simulation is presented in 6.Acknowledgements
No acknowledgement found.References
[1] A. Loktyushin et.al., arXiv:2002.04265 (2020)
[2] J. Schlemper et.al. dAUTOMAP: decomposing AUTOMAP to achieve scalability and enhance performance (2019)
[3] KJ Layton et.al. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magnetic Resonance in Medicine (2017)
[4]C. Lin, Proc. ISMRM #1391 (2001)
5] Henderson, Elizabeth, et al. "A fast 3D look-locker method for volumetric T1 mapping." Magnetic resonance imaging 17.8 (1999)
[6]S. Weinmüller, Proc. ISMRM # 1163 (2021)
Figures