0332
Self-supervised Deep Learning for Rapid Quantitative Imaging1Radiology, Harvard Medical School, Boston, MA, United States, 2Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
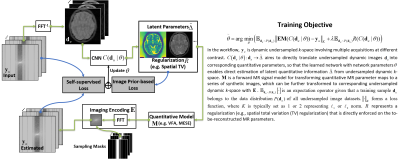
The purpose of this work was to develop a model-guided self-supervised deep learning MRI reconstruction framework called REference-free LAtent map eXtraction (RELAX) for rapid quantitative MR parameter mapping. RELAX eliminates the need for full sampled reference datasets that are required in standard supervised learning. Meanwhile, RELAX also enables direct reconstruction of MR parameter maps from undersampled k-space. Our results demonstrated that the proposed framework produced accurate and robust T1/T2 mapping in accelerated and low-SNR MRI. The good quantitative agreement to the reference method suggests that RELAX allows accelerated quantitative imaging without training with reference data.
INTRODUCTION
Deep learning methods have been successfully used for image reconstruction with promising initial results. To date, most deep learning-based MRI reconstruction techniques are based on a supervised training strategy, which aims to learn the mapping of undersampled images (with artifacts and noises) to corresponding reference images (typically fully sampled). One major challenge of supervised learning, however, is the requirement of abundant training reference images, which can be difficult to acquire. This is even more challenging for quantitative imaging because it typically requires prolonged imaging time and is not routinely implemented in current clinical settings. As a result, the requirement of reference images for network training can greatly restrict the broad applications of supervised learning in quantitative MRI. The purpose of this study was to propose a general self-supervised deep learning reconstruction framework for quantitative MRI. This technique, called REference-free LAtent map eXtraction (RELAX), jointly enforces data-driven and physics-driven training to leverage self-supervised deep learning reconstruction for quantitative MRI.METHODS
(a) Quantitative Imaging: The RELAX framework (Figure 1) performs a learning process to estimate latent parameter maps using Convolutional Neural Network (CNN) mapping and the known MR signal model. The CNN mapping was implemented for converting the undersampled k-space data directly to the parameter maps through learning spatiotemporal information for domain transform. However, unlike standard supervised learning, the RELAX method removed the requirement for the use of reference quantitative maps to guide the network training, rather it ensures that the reconstructed parameter maps from end-to-end CNN mapping produce estimated images matching the acquired images (i.e. model-guided data consistency for self-supervision). Besides, additional regularizations that do not rely on references, as implemented in conventional constrained reconstruction, can be further added to improve the training performance. The RELAX framework was evaluated for reconstructing T1 and T2 from undersampled k-space data. T1 mapping was performed based on variable flip angle (VFA) imaging (1,2). T2 mapping was performed based on multi-echo spin-echo (MESE) imaging (3).(b) Network Implementation: We used a residual U-Net(4) as convolutional encoder/decoder for performing the end-to-end CNN mapping.
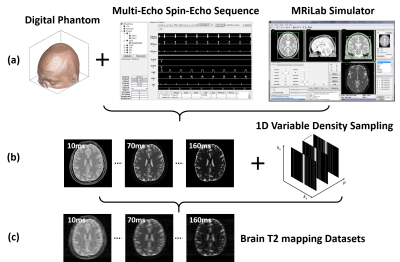
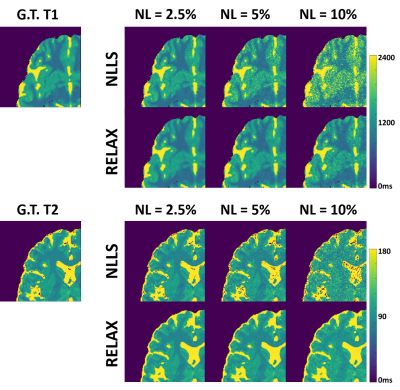
(c) Evaluation: The evaluation of T1 and T2 mapping was performed using image datasets simulated on a realistic MR simulator (open-source MRiLab(5)) on 20 brain models scanned at McGill BrainWeb project(6). A schematic description of the simulation workflow is shown in Figure 2. For VFA-based T1 mapping, the spoiled gradient echo sequence: TR/TE = 8.5/3.9 ms, 8 flip angles = [3, 4, 5, 6, 7, 9, 13, 18]°, number of slices = 40, FOV = 22x22cm2, and matrix = 256×256. For T2 mapping, the multi-echo spin-echo sequence: TR/16TEs = 2500/[10, 20, 30, …, 160] ms, flip angle = 90°, number of slices = 40, FOV = 22x22cm2, and matrix = 256×256. The training/testing data were 18/2 subjects. The performance of RELAX was evaluated for suppressing image noises and/or aliasing artifacts induced by retrospectively undersampling MR k-space. We conducted three experiments. 1) Complex Gaussian noise was added into the simulated noise-free k-space to emulate noise contamination at different noise levels (2.5%, 5%, 10%). 2) Undersampling was simulated by retaining 5% of central k-space to achieve an acceleration rate (R) of 5 using a 1D variable density Cartesian pattern. The sampling pattern was randomly generated for each dynamic frame. 3) Combination of 1) and 2) was used to create noisy undersampled k-space data. The result was compared with standard non-linear least square fit (NLLS) method and cross-validated with ground-truth T1/T2 maps.
RESULTS
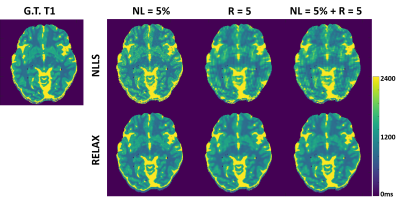
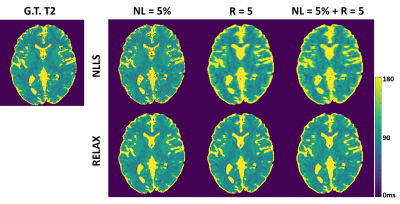
Figure 3 and 4 show representative T1 and T2 maps estimated using RELAX in one simulated brain dataset at three different experimental conditions. On the T1 and T2 maps generated by using the standard NLLS fitting, there is notable noise contamination caused by the added Gaussian noise at 5% noise level (NL) and aliasing artifacts caused by the k-space undersampling at R=5 with simple zero-filling reconstruction. RELAX successfully suppressed the noises and removed the undersampling artifacts through self-supervised deep learning reconstruction, providing image quality that is comparable to the noise/artifact-free ground truth (G.T.) T1 and T2 maps. Figures 3 and 4 demonstrate the generality of RELAX to model different quantitative imaging process by incorporating corresponding signal models based on self-supervised learning. Figure 5 shows the performance of RELAX in different noise levels. Generally, RELAX generated acceptable T1 or T2 parameters at different noise conditions due to the inherent noise suppression in CNN training and the additional spatial TV constraint.DISCUSSION AND CONCLUSION
The direct image-to-parameter transformation enabled by the model-augmented CNN mapping with self-supervised learning provides a promising approach for efficient and robust MR parameter mapping. The proposed framework produced accurate solutions for parameters in both accelerated and low-SNR imaging applications. This new framework provides good quantitative agreement with standard NLLS while used only undersampled data or noise-contaminated data. This new technique may be particularly useful in quantitative MRI applications where fully sampled reference images are challenging to acquire. It holds great potential to improve imaging speed in quantitative MRI and to help the clinical translation of quantitative imaging. In addition, the proposed framework can be extended to support other quantitative imaging with the incorporation of proper MR signal models.Acknowledgements
No acknowledgement found.References
1. Wang HZ, Riederer SJ, Lee JN. Optimizing the precision in T1 relaxation estimation using limited flip angles. Magn. Reson. Med. 1987;5:399–416 doi: 10.1002/mrm.1910050502.
2. Venkatesan R, Lin W, Haacke EM. Accurate determination of spin-density and T1 in the presence of RF- field inhomogeneities and flip-angle miscalibration. Magn. Reson. Med. 1998;40:592–602 doi: 10.1002/mrm.1910400412.
3. Meiboom S, Gill D. Modified Spin‐Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958;29:688–691 doi: 10.1063/1.1716296.
4. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In: Navab N, Hornegger J, Wells WM, Frangi AF, editors. Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2015: 18th International Conference, Munich, Germany, October 5-9, 2015, Proceedings, Part III. Cham: Springer International Publishing; 2015. pp. 234–241. doi: 10.1007/978-3-319-24574-4_28.
5. Liu F, Velikina J V., Block WF, Kijowski R, Samsonov AA. Fast Realistic MRI Simulations Based on Generalized Multi-Pool Exchange Tissue Model. IEEE Trans. Med. Imaging 2017;36:527–537 doi: 10.1109/TMI.2016.2620961.
6. Aubert-Broche B, Griffin M, Pike GB, Evans AC, Collins DL. Twenty new digital brain phantoms for creation of validation image data bases. IEEE Trans. Med. Imaging 2006;25:1410–1416 doi: 10.1109/TMI.2006.883453.
Figures